Professor Zhengxiao Guo from the Department of Chemistry at The University of Hong Kong (HKU) and Professor Junwang Tang, now at the Department of Chemical Engineering at Tsinghua University, have collaborated to develop a highly active and selective catalytic material that can effectively transform methane, a powerful greenhouse gas, into formaldehyde, an indispensable chemical, in a waste-free manner.
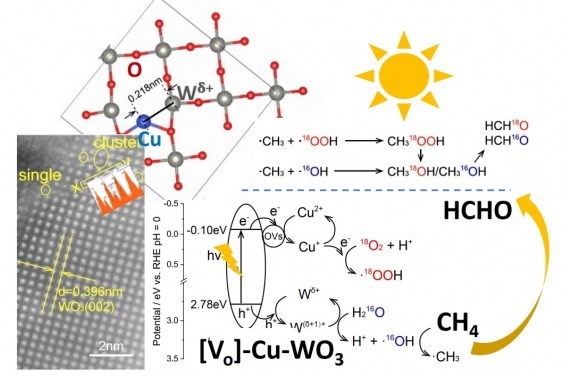
Selective methane conversion to formaldehyde over a [Vo]-Cu-WO3 photocatalyst. Image Credit: Nature Communications
This novel material, derived from tungsten trioxide (WO3 catalyst), has a dual active site composed of copper and tungsten atomic species that work together to enable an efficient and selective conversion process.
Under visible light, the conversion process can achieve nearly 100% selectivity, avoiding undesired byproducts and increasing efficiency, rendering it an eco-friendly option to current production methods. The research was recently published in the prominent journal Nature Communications.
Change the Unchanged: Methane Conversion
Methane, the main component of natural gas, is a common carbon source for many chemicals. Nonetheless, it is a strong greenhouse gas, with a global warming potential of more than 70 times that of carbon dioxide. Catalytic methane conversion (turning methane into other chemicals) thus represents a huge possibility for obtaining net-zero energy and chemical supply while also addressing environmental issues.
Methane, on the other hand, is an extremely stable molecule that is resistant to activation, especially in mild or ambient conditions. Thus, establishing high activity and selectivity in methane conversion is a considerable problem, and many chemists regard selective activation of the intermolecular carbon-hydrogen bond as one of the most elusive “holy grails” in catalysis.
In contrast, formaldehyde is a high-volume commodity chemical with a market value of USD 8 billion and a compound annual growth rate (CAGR) of 5.7%. It is utilized in a variety of household, commercial, aviation, medical, and automotive industries, as well as a vital precursor for melamine, urea-formaldehyde, and phenolic resins.
Formaldehyde is also used safely to make vaccines, anti-infective drugs, and hard-gel capsules. It is currently manufactured via methanol oxidation-dehydrogenation utilizing silver or metal-oxide catalysts at high reactor temperatures of approximately 500–600 °C, resulting in significant CO2 emissions and energy penalties.
Harnessing Sunlight to Convert Methane
The group uncovered a novel approach to convert methane gas into formaldehyde utilizing sunlight in their research. They discovered that a synergistic ensemble comprising atomically dispersed copper and partly reduced tungsten species over tungsten oxide functioned extremely well, enabling remarkable photocatalytic methane conversion to formaldehyde under ambient visible light.
The technique outperformed previously reported photocatalysts by nearly 100% selectivity and high conversion efficiency (with a turnover frequency, TOF = 8.5×106 μmol (HCHO)·g-1(cocatalyst)·h-1).
Researchers, through mechanistic analysis, discovered that the copper helped to move electrons around and form reactive molecular species, while the tungsten assisted in activating the methane gas. The copper, in particular, worked as electron acceptors, promoting photo-induced electron transfer from the conduction band to dioxygen, resulting in reactive hydroperoxyl radicals (HOO).
Meanwhile, the nearby tungsten atoms with a partial positive charge served as hole acceptors. Water’s preferred adsorption and activation site generated hydroxyl radicals, which effectively converted methane to methyl radicals. The synergy of the adjacent dual active sites significantly improves the conversion process’s overall efficiency and selectivity.
This discovery provides the path for continued study and development of new photocatalysts for a wide range of chemical conversions, supporting more sustainable and efficient chemical processes.
Solar conversion of methane is highly desirable for both low-carbon and high-value-added chemical syntheses. However, product selectivity and production efficiency are key to success. This requires an in-depth understanding of the conversion mechanism, careful design of the catalyst, and complementary techniques to confirm its performance—a good case of multidisciplinary tasks that require strong collaborative dedication. That’s exactly what the team has managed to do - with much value-added outcome!
Zhengxiao Guo, Study Corresponding Authors and Professor, The University of Hong Kong
Journal Reference
Luo, L., et al., (2023). Nearly 100% selective and visible-light-driven methane conversion to formaldehyde via. single-atom Cu and Wδ+. Nature Communications. doi.org/10.1038/s41467-023-38334-7.