Aluminum-ion batteries are viewed as a viable replacement for traditional batteries, which rely on limited and difficult-to-recycle basic elements like lithium. This is due to the fact that lithium is more expensive and safer than aluminum, which is one of the most prevalent materials in the Earth’s crust, and aluminum is also easier to recycle.
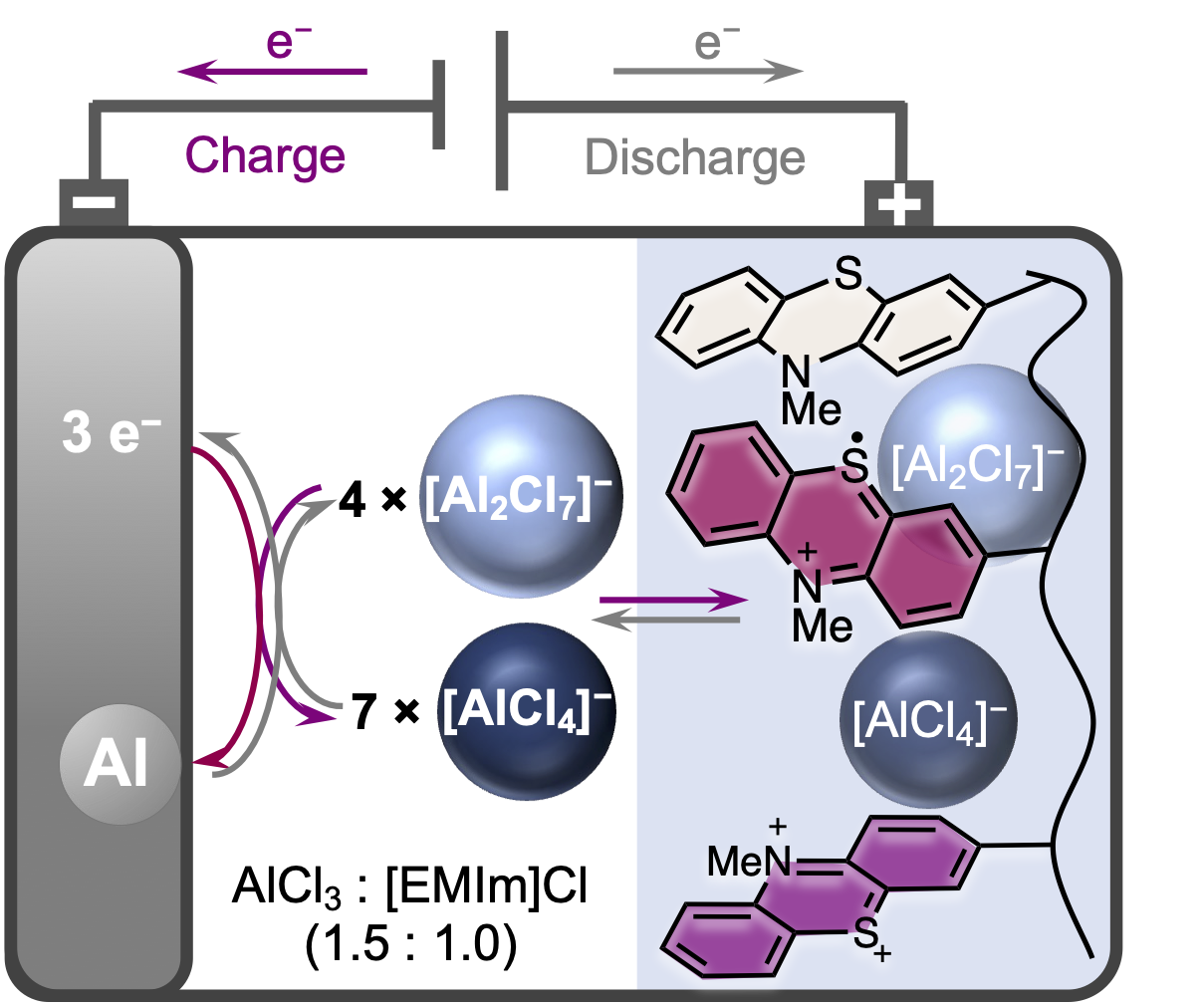 The schematic diagram of the battery shows the redox process in which the electrode material is oxidized and aluminate anions are deposited. Image Credit: Birgit Esser (CC BY-NC 3.0)
The schematic diagram of the battery shows the redox process in which the electrode material is oxidized and aluminate anions are deposited. Image Credit: Birgit Esser (CC BY-NC 3.0)
However, due to a lack of suitable electrode materials that offer adequate storage capacity, the development of such aluminum-ion batteries is still in its infancy.
A positive electrode material made of an organic redox polymer based on phenothiazine has now been created by a research team led by Prof. Dr. Birgit Esser of the University of Ulm, Prof. Dr. Ingo Krossing, and Prof. Dr. Anna Fischer of the University of Freiburg under the direction of Gauthier Studer.
In the experiment, aluminum batteries with this electrode material were able to store 167 milliampere hours per gram (mAh/g), which was a record-breaking amount of energy. Thus, the organic redox polymer outperforms graphite, which has traditionally been utilized as electrode material in batteries. The findings were published in Energy & Environmental Science.
Electrode Material Inserts Complex Aluminum Anions
During battery charging, the electrode material is oxidized, absorbing complex aluminate anions. During charging, two [AlCl4]– anions are reversibly inserted into the organic redox polymer poly(3-vinyl-N-methylphenothiazine).
The scientists mixed aluminum chloride with the ionic liquid ethylmethylimidazolium chloride to use it as an electrolyte.
The study of aluminum batteries is an exciting field of research with great potential for future energy storage systems. Our focus lies on developing new organic redox-active materials that exhibit high performance and reversible properties. By studying the redox properties of poly(3-vinyl-N-methylphenothiazine) in chloroaluminate-based ionic liquid, we have made a significant breakthrough by demonstrating for the first time a reversible two-electron redox process for a phenothiazine-based electrode material.
Gauthier Studer, Doctoral Student, Institute for Organic Chemistry II and Advanced Materials, University of Ulm
After 5,000 Charge Cycles at 10 C, Battery Retains 88 Percent of its Capacity
At potentials of 0.81 and 1.65 volts, poly(3-vinyl-N-methylphenothiazine) deposits the [AlCl4] anions and offers specific capacities of up to 167 mAh/g. In comparison, the graphite used as the electrode material in aluminum batteries has a discharge capacity of 120 mAh/g.
The battery that the study team demonstrated still has 88 percent of its capacity at 10 C, or at a charge and discharge rate of 6 minutes, even after 5,000 charge cycles. The battery restores to its previous capacity at a lower C rate, or a longer charge and discharge time.
With its high discharge voltage and specific capacity, as well as its excellent capacity retention at fast C rates, the electrode material represents a major advance in the development of rechargeable aluminum batteries and thus of advanced and affordable energy storage solutions.
Birgit Esser, Chair of Organic Chemistry, Institute of Organic Chemistry II and Advanced Materials, University of Ulm
The Deutsche Bundesstiftung Umwelt, the bwForCluster JUSTUS 2, the Eva Mayr-Stihl-Stiftung (Saltus!), the Land Baden-Württemberg (bwHCP), and the German Research Foundation (DFG) provided funding for the project (project AMPERE within SPP 2248 - Polymer-based Batteries, POLiS - EXC 2154, and livMatS - EXC 2193).
Journal Reference:
Studer, G., et al. (2023) On a high-capacity aluminium battery with a two-electron phenothiazine redox polymer as positive electrode. Energy & Environmental Science. doi:10.1039/D3EE00235G.