Carbon dioxide emissions captured could be converted into valuable chemicals using a selective process powered by renewable energy. By performing the reaction at high pressure, CO2 can be electrochemically transformed into a specific product.
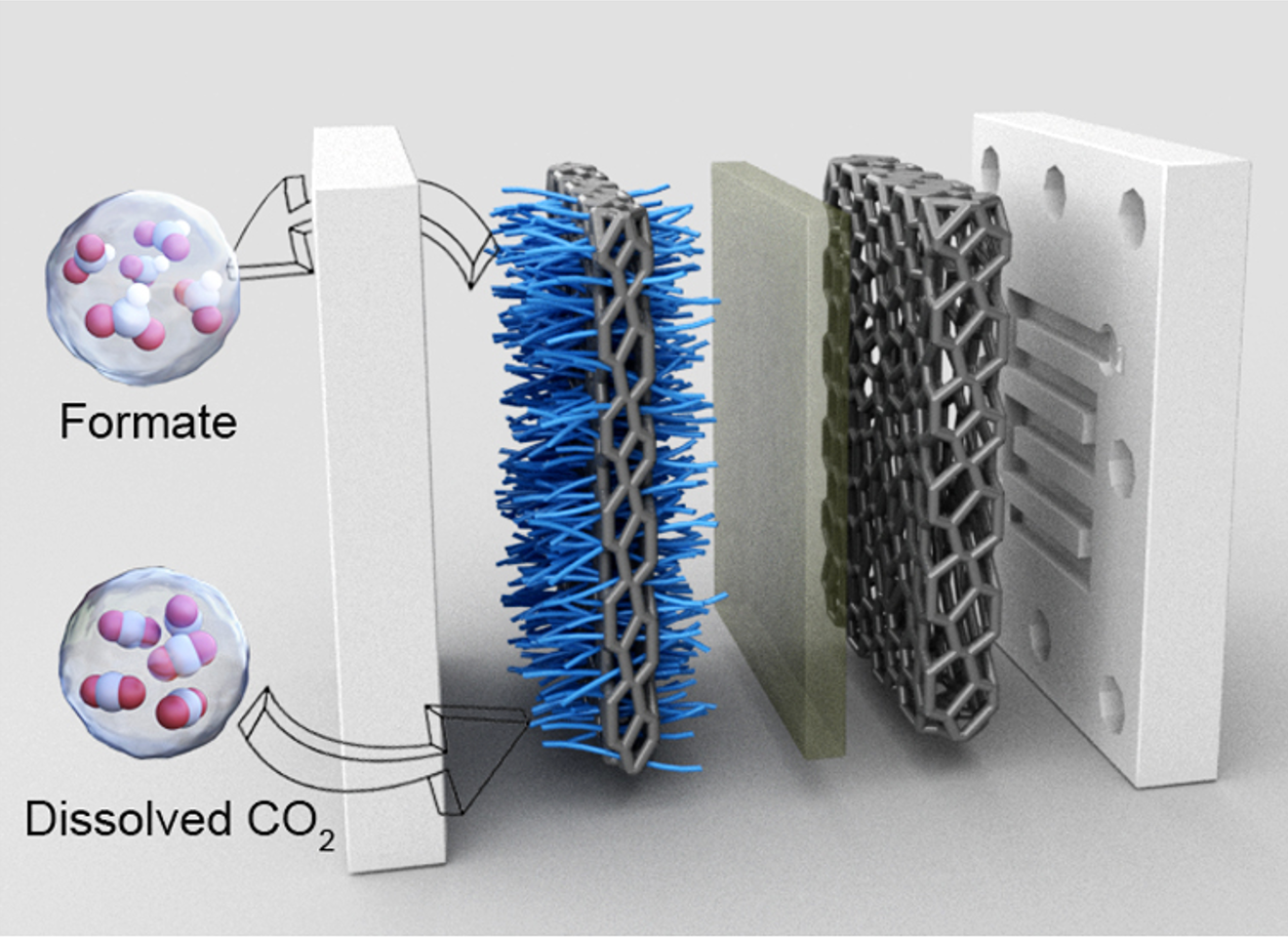 Under specific pressurized conditions, carbon dioxide can be catalytically transformed into formate in a high-pressure narrow-gap aqueous flow cell. Image Credit: 2023, Huang et al.
Under specific pressurized conditions, carbon dioxide can be catalytically transformed into formate in a high-pressure narrow-gap aqueous flow cell. Image Credit: 2023, Huang et al.
In my research, we use electricity to convert CO2 into fuels.
Xu Lu, Mechanical Engineer, King Abdullah University of Science and Technology
The researchers investigated a method known as electrochemical CO2 reduction (CO2R), that could convert waste gas into a variety of potential fuel molecules. Depending on the catalyst, compounds such as formate, methanol, and ethanol can be produced.
The majority of investigations up to this point have examined CO2R at ambient pressure levels. However, CO2 is frequently caught, moved, and stored under high pressure in the industrial sector.
Since industrial CO2 sources are often pressurized, the direct electrochemical reduction of high-pressure CO2 would be of significant value to industry.
Liang Huang, Postdoctoral Researcher, King Abdullah University of Science and Technology
So, Lu, Huang, and their colleagues investigated what occurs when the reaction is done at 50 bar pressure, which is more typical of industrial circumstances. At this pressure, the reaction's result was considerably changed.
Huang added, “To our surprise, we found that catalysts including copper, silver, gold and tin, which exhibited different CO2R selectivities under ambient pressure, all became selective for formate production at high pressure.”
Converting CO2 into a single product is preferable to producing a combination.
Since most analytical methods function at ambient pressure, it is not simple to understand reaction processes occurring inside a high-pressure reactor. Gaetano Magnotti, an expert in high-pressure combustion processes and sophisticated laser diagnostics and imaging, worked with Lu’s team to evaluate the outcome of the reaction.
Lu added, “We used the high-power system to shoot a laser into our high-pressure electrochemical reactor.”
The group measured the reactant species that appeared on the electrode surface during CO2R using this configuration.
“Together with Edward Sargent’s group from the University of Toronto, we then developed a theoretical model to discover the key factors governing the selectivity shift toward formate,” Huang further stated.
The researchers discovered that under high pressure, more CO2 covers the catalyst surface, increasing the reaction rate and eventually changing the reaction selectivity. They then demonstrated that they could raise formate selectivity much more.
“We found that under pressure, a competing reaction called the hydrogen evolution reaction (HER) is the primary factor limiting CO2R selectivity. We modified our copper electrode surface with a layer capable of inhibiting HER, enhancing formate selectivity,” Huang added.
According to Huang, the group is now examining CO2R in several high-pressure reactor designs, such as flow cells and membrane electrode assembly (MEA) reactors, in an effort to determine their potential utility to industry.
Journal Reference:
Huang, L., et al. (2023) Pressure dependence in aqueous-based electrochemical CO2 reduction. Nature Communications. doi:10.1038/s41467-023-38775-0