Supramolecular polymers (SPs) are expected to be used as highly recyclable polymer materials. However, their application is hindered by the low solubility of monomer compounds associated with the formation of a crystalline polymorphic state. To address this, researchers from Japan employed a supramolecular coformer capable of deteriorating the structural order of the insoluble polymorph through co-aggregation with the parent molecule. Mixing the two molecules resulted in significant solubility enhancement of the parent compound, leading to the preparation of SPs at high concentrations. Going ahead, this approach can contribute to the large-scale production of SPs for evaluating their functionalities and applications.
 (a) An insoluble crystalline polymorphic state inhibits supramolecular polymerization at high concentrations. (b) Mixing the coformer molecule with the parent monomer in the solidification process deteriorates the structural order of the crystalline polymorphic state, resulting in the formation of a co-aggregated state with high solubility. Image Credit: Atsushi Isobe from Chiba University, Japan
(a) An insoluble crystalline polymorphic state inhibits supramolecular polymerization at high concentrations. (b) Mixing the coformer molecule with the parent monomer in the solidification process deteriorates the structural order of the crystalline polymorphic state, resulting in the formation of a co-aggregated state with high solubility. Image Credit: Atsushi Isobe from Chiba University, Japan
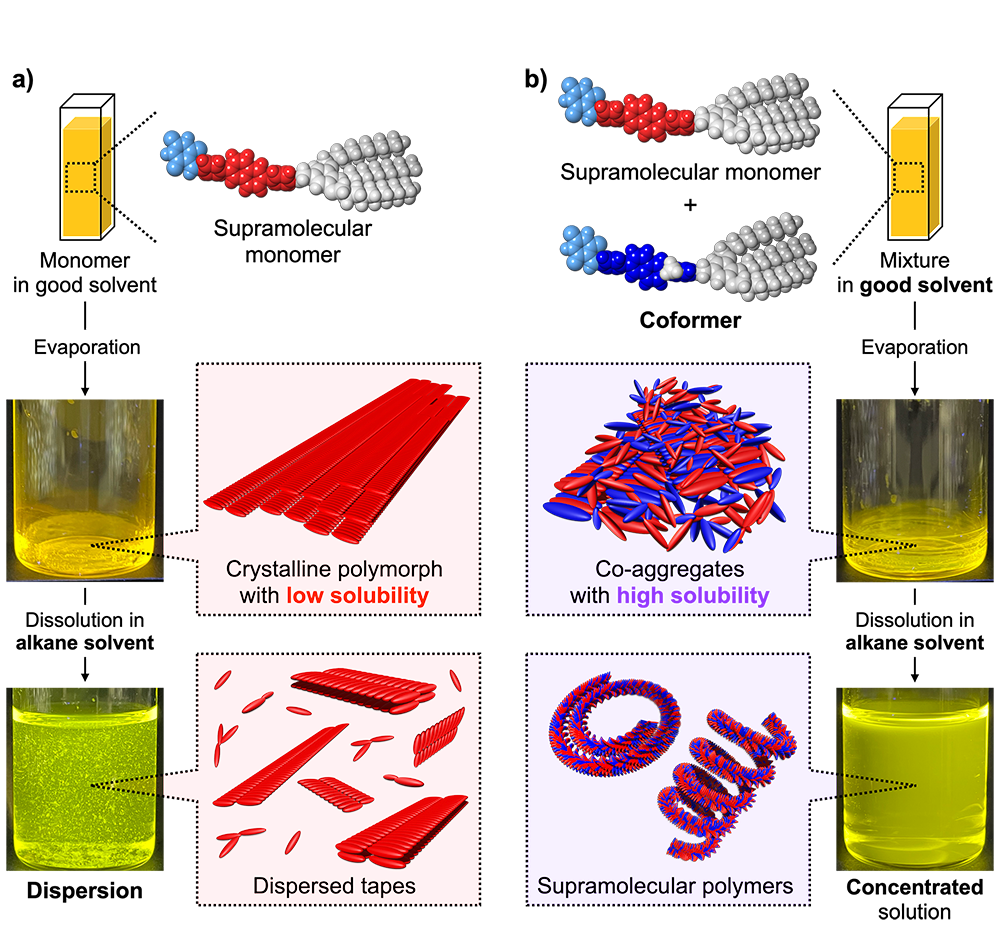
Supramolecular polymers (SPs) are molecular assemblies composed of non-covalently bonded small molecules. They show high recyclability originating from their dynamic nature of monomer binding, which is different from covalent polymers with non-biodegradable nature. The small repeating units that form SPs, called monomers, are specifically designed to construct multiple non-covalent bonds to enhance the stability of the resulting SPs. Such monomers can be organized into structurally distinct assemblies by forming various molecular arrangements depending on monomer concentration, solvent composition, and temperature. This formation of versatile self-assembled structures from a single molecule is referred to as polymorphism.
However, polymorphism potentially leads to a reduction in the solubility of molecules by forming a crystalline polymorphic state. This issue is prevalent in various chemical industries, such as pharmaceuticals, where the solubility of a drug molecule affects the functionalities of the drug molecule. To overcome this problem, the use of a “coformer” molecule has been known to be one of the approaches in the pharmaceutical industry. The coformer molecule can inhibit the formation of the crystalline polymorphic states through co-aggregation with the parent molecule, thereby enhancing the solubility of the drug molecule.
Inspired by this approach, a research team from Japan, led by Professor Shiki Yagai from the Institute for Advanced Academic Research at Chiba University, has recently explored a novel supramolecular coformer approach. “We applied this approach to a supramolecular monomer that can form an insoluble crystalline polymorphic state,” explains Dr. Yagai. Their paper, published in Angewandte Chemie International Edition on September 22, 2023, is co-authored by Atsushi Isobe from the Graduate School of Science and Engineering at Chiba University and Dr. Takashi Kajitani from the Open Facility Center at Tokyo Institute of Technology.
The team has investigated SPs formed from a π-conjugated monomer functionalized with a barbituric acid multiple hydrogen-bonding unit represented by compound 1. This supramolecular monomer was poorly soluble by forming a crystalline polymorphic state during the preparation procedures of SPs. Therefore, the researchers newly designed compound 2 with a slightly modified molecular structure of the parent compound 1 as a supramolecular coformer. The compound 2 was highly soluble because the molecular modification effectively prevented the formation of the crystalline polymorphic state as observed for the parent compound 1. Upon mixing the two compounds, the researchers demonstrated significantly improved solubility of the parent monomer 1, without affecting its ability to form SPs.
The use of this supramolecular coformer thus helped in overcoming issues related to crystallinity and enabled the preparation of SPs at high concentrations. Additionally, by utilizing the reversible nature of monomer bindings, the coformer molecules could be separated from the resulting SPs.
Emphasizing the importance of these findings, Dr. Yagai says, “Use of coformer can facilitate the large-scale production of SPs, leading to the evaluation of their functionalities. The application of supramolecular polymers can contribute to the production of novel plastic materials showing high recyclability originating from the reversibility of monomer bindings and recycle them with lower energy consumption.”
In the long run, this can pave the way for the development of improved recyclable plastics for a sustainable future.