Zhongyuan University of Technology researchers have suggested a novel approach for effectively converting nitrate, a common water contaminant, back into useful ammonia.
 Scientists from Zhongyuan University of Technology developed a novel path to efficiently convert nitrate to ammonia using metal-added polyoxometalate as the catalyst under mild operating conditions. Image Credit: Zhihui Ni, Henan Key Laboratory of Functional Salt Materials, Zhongyuan University of Technology
Scientists from Zhongyuan University of Technology developed a novel path to efficiently convert nitrate to ammonia using metal-added polyoxometalate as the catalyst under mild operating conditions. Image Credit: Zhihui Ni, Henan Key Laboratory of Functional Salt Materials, Zhongyuan University of Technology
The team’s newly published study described a unique method that uses metal-added polyoxometalate as the catalyst under mild operating conditions to effectively convert nitrate to ammonia.
Polyoxometalates published the study on December 8th, 2023.
Numerous techniques have been used in the last few decades to reduce nitrate nitrogen, a factor in the poisoning of groundwater. Prior research has demonstrated that chemical reduction is capable of not only reducing or eliminating nitrogen nitrates but also transforming hazardous pollutants into ammonia, a significant synthetic industrial chemical utilized on a global scale.
Ammonia is widely used in numerous sectors and is easy to liquefy and transport. It also has an exceptionally high energy density. The Haber-Bosch process, which dates back a century, uses an iron metal catalyst to react with hydrogen at high temperatures and pressures to transform atmospheric nitrogen into ammonia.
However, the “fixation” process requires high temperatures and pressures, which are energy-intensive and emit a significant quantity of greenhouse gases.
We should find high-efficiency, environmentally friendly methods for reducing nitrogen to ammonia under mild conditions.
Zhihui Ni, Study Author, Zhongyuan University of Technology
Scientists have been working on many mild nitrogen reduction methods in recent years to replace the Haber-Bosch process. These methods include microbial fuel cells, photocatalysis, and electrocatalysis. Of these, the electrochemical nitrate reduction process is a viable synthetic substitute that may be used to manufacture ammonia sustainably while simultaneously removing nitrate from water under moderate operating conditions.
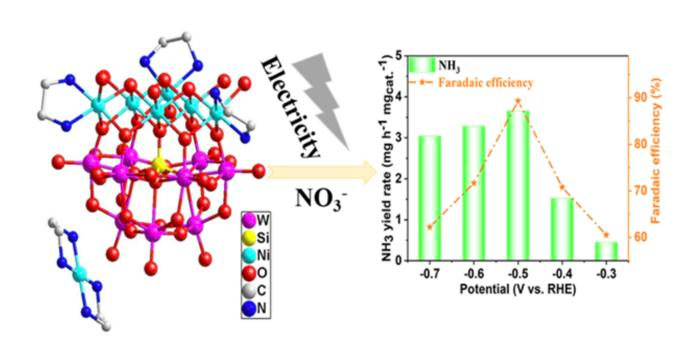
Based on this, the researchers created two nickel-added polyoxometalates (POMs), a family of metal-oxide clusters with special physicochemical characteristics that enable them to be extremely helpful in driving chemical reactions using electrical energy.
POMs can act as catalysts to degrade organic contaminants in wastewater and lower carbon dioxide levels because of the stability of their molecular structures and reversible redox characteristics. POMs are also capable of catalyzing bond formation and other basic organic reactions.
The nickel compounds’ structures were described by the study team, who then hydrothermally manufactured them to test them at extreme pressure. The electrochemical nitrate reduction reaction experiments were carried out using an electrochemical workstation.
Ni added, “To evaluate the practicality of the nitrate reduction under real operating conditions, we conducted the electrocatalytic tests over a wide range of nitrate concentrations.”
Along with other criteria, they investigated the electrocatalysts’ stability, ammonia production rate, and Faraday efficiency.
High-efficiency electrochemical catalytic nitrogen reduction to ammonia is demonstrated by the results.
“This discovery creates a novel path for manufacturing highly effective electrochemical nitrate reduction reaction electrocatalysts using metal-added polyoxometalate as the catalyst in ambient settings. The research findings offer practical advice for creating effective electrocatalytic catalysts,” Ni further added.
The study team intends to investigate this technique for producing efficient electrocatalytic catalysts in more detail in the future.
The University Natural Science Foundation of Zhongyuan Institute of Technology and the Henan Province Natural Science Foundation both provided funding for the study.
Ning Liu, Chunhui Zhao, and Liwei Mi from Zhongyuan University of Technology are among the other contributors.
Journal Reference:
Ni, Z., et. al. (2023) Hexanuclear nickel-added silicotungstates as high-efficiency electrocatalysts for nitrate reduction to ammonia. Polyoxometalates. doi:10.26599/POM.2023.9140044