Gears play a fundamental role in the functionality of everyday machines, offering a means to regulate motion intensity or direction. Similar to shifting gears in a car, this capability enhances the versatility of machines.
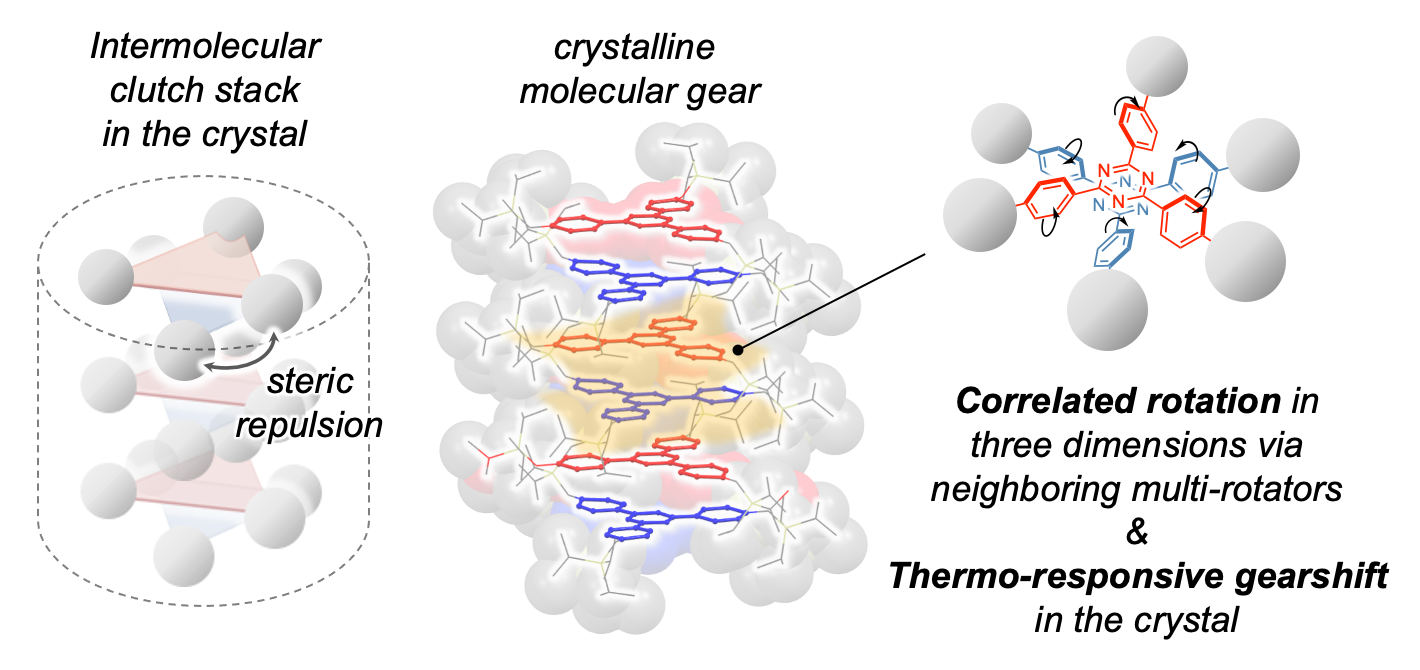 (Left) Schematic of the clutch stack arrangement. (Middle) Structure of the molecular gears in the clutch stack. (Right) Rotational direction of two adjacent molecular gears. Image Credit: Mingoo Jin, et al. Journal of the American Chemical Society.
(Left) Schematic of the clutch stack arrangement. (Middle) Structure of the molecular gears in the clutch stack. (Right) Rotational direction of two adjacent molecular gears. Image Credit: Mingoo Jin, et al. Journal of the American Chemical Society.
A team led by researchers at the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD) at Hokkaido University has introduced a novel design strategy for creating molecular-sized gears within crystals. They have achieved the first instance of controllable molecular gear shifting in a solid material.
The team developed a crystalline material housing gear-like molecules capable of reversible shifts between two types of motion. This innovative design approach is a blueprint for developing adaptable and innovative materials.
In their study, researchers employed a gear-shaped molecule known as triaryltriazine, featuring a central triazine ring with three phenylene rings attached, resembling the teeth of a gear.
To create a distinctive arrangement, they attached sizable, fixed molecules to the phenylene rings, resulting in a "clutch stack" configuration. This arrangement caused adjacent triaryltriazine molecules to rotate 60° from each other, deviating from the conventional stacking in the same orientation.
The design of the clutch stack was inspired by the mechanical machinery system of the clutch in a car.
Mingoo Jin, Associate Professor, Hokkaido University
The fixed molecules attached to the structure provided sufficient space, allowing the three phenylene rings to undergo a flapping motion between two positions. With the clutch stack arrangement of the triaryltriazine molecules, neighboring molecules could interlock as the phenylene rings rotated, resembling the engagement of interlocking gears. Consequently, this led to a synchronized motion of all the molecules within the stack.
Upon raising the temperature beyond a specific threshold, a distinct correlated motion was observed, characterized by a 180° rotation of the phenylene rings. This shift in motion was linked to a phase transition in the crystal, introducing increased space between adjacent molecules. The expanded space facilitated greater freedom for the phenylene rings to undergo the 180° rotation.
The researchers discovered that the observed change in motion could be reversed by cooling the crystal, representing the first instance of controllable molecular motion in a solid.
The fine-tuning of the molecular gearshift effect was achieved by modifying the size and structure of the stationary molecule connected to the gear molecule. This adjustability holds significant promise for the advancement of novel functional materials, capitalizing on the capabilities of crystalline molecular machines.
The next direction for our research would be using geared molecular motion in crystals to manipulate different physical properties of solid-state materials, such as light emission or thermal behavior.
Mingoo Jin, Associate Professor, Hokkaido University
Clutch-stack-driven molecular gears
Phenylene rings of the clutch-stacked triaryltriazine molecular gears rotate 180°, perpendicular to the molecular plane, interacting with phenylene rings of neighboring molecules in a gear-like fashion, resulting in a correlated molecular motion. Video Credit: Mingoo Jin, et al. Journal of the American Chemical Society.
Journal Reference:
Jin, M., et al. (2023). A Steric-Repulsion-Driven Clutch Stack of Triaryltriazines: Correlated Molecular Rotations and a Thermoresponsive Gearshift in the Crystalline Solid. Journal of the American Chemical Society. doi.org/10.1021/jacs.3c08909.