Feb 16 2024
One of materials science’s big challenges is to create and find novel materials that satisfy global objectives like Net Zero.
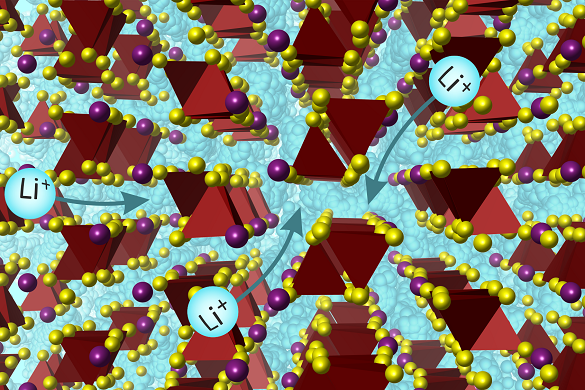 Image represents the lithium ions (in blue) moving through the structure. Image Credit: University of Liverpool
Image represents the lithium ions (in blue) moving through the structure. Image Credit: University of Liverpool
According to a study published in the journal Science, a solid that conducts lithium (Li) ions quickly has been found by researchers at the University of Liverpool. These Li-ion electrolytes are crucial parts of the rechargeable batteries that run a lot of modern devices, including electric vehicles.
The new material, which is composed of components that are naturally occurring and non-toxic, has a high enough Li-ion conductivity to replace the liquid electrolytes used in existing Li-ion battery technology, increasing energy capacity and safety.
The multidisciplinary research team from the university designed the material using a transformational scientific method. They synthesized the material in the lab, identified its structure (the arrangement of atoms in space), and showed it in a battery cell.
The new substance is one of just a few solid materials that have Li-ion conductivity high enough to replace liquid electrolytes, and it acts in a novel way due to its structure.
It was discovered using a combined computational and experimental methodology that employed artificial intelligence (AI) and physics-based computations to complement decisions made by university chemistry specialists.
Based on the novel insights provided by the study, the new material offers a platform for optimizing chemistry to improve its inherent qualities and identify alternative materials.
According to the researchers:
This research demonstrates the design and discovery of a material that is both new and functional. The structure of this material changes previous understanding of what a high-performance solid-state electrolyte looks like. Specifically, solids with many different environments for the mobile ions can perform very well, not just the small number of solids where there is a very narrow range of ionic environments. This dramatically opens up the chemical space available for further discoveries.
They continued, “Recent reports and media coverage herald the use of AI tools to find potentially new materials. In these cases, the AI tools are working independently and thus are likely to recreate what they were trained on in various ways, generating materials that may be very similar to known ones.”
In contrast, this discovery research paper shows that AI and computers marshaled by experts can tackle the complex problem of real-world materials discovery, where we seek meaningful differences in composition and structure whose impact on properties is assessed based on understanding. Our disruptive design approach offers a new route to discovery of these and other high-performance materials that rely on the fast motion of ions in solids.
Matt Rosseinsky, Professor, Department of Chemistry, University of Liverpool
The study was published in Science.
Researchers from the Department of Chemistry, Materials Innovation Factory, Leverhulme Research Centre for Functional Materials Design, Albert Crewe Centre, Stephenson Institute for Renewable Energy, and School of Engineering at the University of Liverpool collaborated on the study.
The Faraday Institution, the Leverhulme Trust, and the Engineering and Physical Sciences Research Council (EPSRC) provided funding for the study.
Journal Reference:
Han, G., et. al. (2024) Superionic lithium transport via multiple coordination environments defined by two-anion packing. Science. doi:10.1126/science.adh5115