Propylene is a vital petrochemical raw material, trailing only behind ethylene in significance. With demand continuously on the rise, there is a pressing need to explore alternative production methods. Among these, propane dehydrogenation (PDH) is widely regarded as the most promising solution.
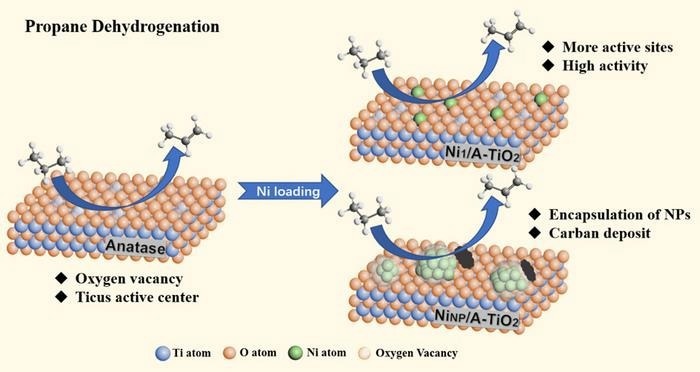
Ni single-atom catalysts with extremely low Ni loading exhibit very high intrinsic activity in terms of propane conversion and decent stability in propane dehydrogenation reactions. The single Ni atoms mainly play an active center rather than promoting the formation of oxygen vacancies or coordinated unsaturated Ti ion sites of TiO2 support. Image Credit: Chinese Journal of Catalysis
Due to its cost-effectiveness and eco-friendly attributes, Ni-based catalysts have garnered significant attention from researchers across diverse catalytic applications, including hydrogenation, methane reforming, electrochemical processes, and photocatalysis. However, there has been limited exploration of nickel's role in alkane dehydrogenation at elevated temperatures.
This is primarily due to the tendency of nickel species to undergo reduction to form metallic nickel nanoparticles (NPs) under harsh reaction conditions. Such transformations can lead to extensive dehydrogenation and compromised selectivity in the process.
The emergence of single-atom catalysts (SACs) represents a groundbreaking development in catalysis, finding widespread utility across various catalytic processes. However, their application in the dehydrogenation of light hydrocarbons at high temperatures has been somewhat limited thus far.
In propane dehydrogenation, the activation of C-H bonds exhibits little sensitivity to the catalyst structure. However, undesirable side reactions such as hydrolysis, isomerization, and coking are typical structure-sensitive reactions that require the participation of multiple metal atoms. Consequently, SACs featuring isolated dispersed metal active centers offer clear advantages in suppressing these side reactions, positioning them as promising candidates for catalytic alkane dehydrogenation.
Anatase TiO2-supported Ni single-atom catalyst (Ni1/A-TiO2) recently showed superior intrinsic activity and propylene selectivity as well as much better stability than the corresponding Ni nanoparticle (NP) catalyst (NiNP/A-TiO2) in PDH reaction at 580 °C, according to a research team led by Professor Botao Qiao from Dalian Institute of Chemical Physics, Chinese Academy of Sciences.
The rate of propylene production on Ni1/A-TiO2 was about 1.96 molC3H6·gNi-1·h-1, above 65 times than that of NiNP/A-TiO2 sample (0.03 molC3H6·gNi-1·h-1).
Combining HAADT-STEM, in-situ CO-DRIFTS, in-situ XPS, and XAS characterizations, researchers confirmed that the Ni SAC mainly contains individual Ni atoms singly dispersed on the support in a positive Ni (II) valence state. These atoms primarily serve as active centers rather than promoting the formation of coordinated unsaturated Ti ion sites.
In addition, as a result of strong metal-support interaction between Ni NPs and TiO2 carriers during reduced conditions, the Ni nanoparticles sites were encapsulated by TiOx overlayer (~2 nm thick), thus displaying lower initial propane conversion and lower durability.
This work highlights the advantage of single-atom catalysts with isolated active sites in PDH reactions and provides a reference for future research on the preparation and application of SACs. The results were published in the Chinese Journal of Catalysis.
Journal Reference:
Zhang, Q., et al. (2024) Catalytic propane dehydrogenation by anatase-supported Ni single-atom catalysts. Chinese Journal of Catalysis. doi.org/10.1016/s1872-2067(23)64584-x