Reviewed by Lexie CornerApr 4 2024
Phosphoric acid and platinum catalyst interactions in high-temperature PEM fuel cells are more intricate than previously thought. Utilizing tender X-Rays, BESSY II experiments have unraveled the multiple oxidation processes occurring at the interface between platinum and electrolyte. The findings suggest that changes in humidity could have an impact on a few of these processes, extending the lifespan and improving fuel cell efficiency.
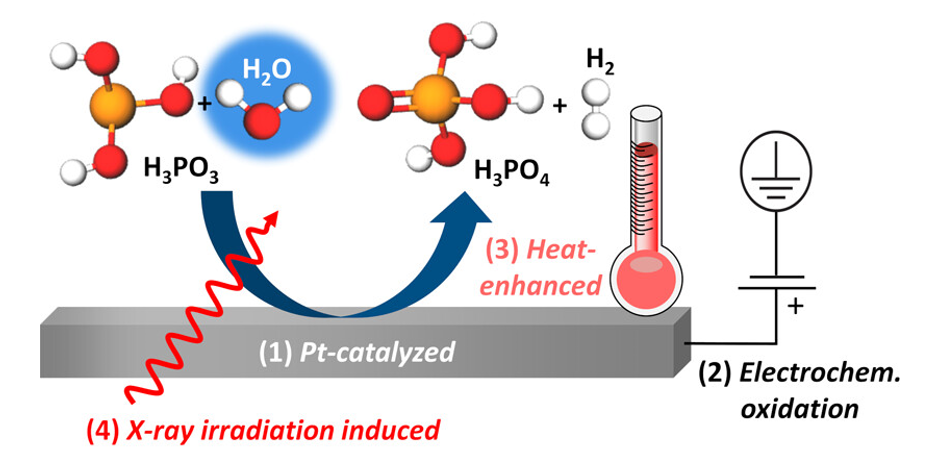 The illustration shows four different oxidation pathways (1-4) of aqueous phosphoric acid (H3PO3), which XANES could elucidate at BESSY II. All these reactions depend on the humidity present. Image Credit: HZB
The illustration shows four different oxidation pathways (1-4) of aqueous phosphoric acid (H3PO3), which XANES could elucidate at BESSY II. All these reactions depend on the humidity present. Image Credit: HZB
Hydrogen fuel cells use distinct interactions between hydrogen fuels and oxidizing agents (oxygen) to transform chemical energy from hydrogen into electrical energy. For micro-stationary electricity sources, high-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs) are a desirable type of hydrogen fuel cell.
One downside of these HT-PEMFCs is that the phosphoric acid (H3PO4) proton conductor leaches out of the H3PO4-doped polybenzimidazole membrane, poisoning the platinum catalyst. Recent investigations have revealed further problems during the operation of the HT-PEMFC, where some H3PO4 can be converted to H3PO3, further poisoning the platinum catalysts and resulting in a considerable loss of performance.
Complex Processes and Interactions
An earlier study by Prof. Dr. Marcus Bär’s team demonstrated that opposite processes occur at the interface between Pt and aqueous H3PO3 and that the interactions between the platinum catalyst and the H3PO3/H3PO4 are very complex. While H3PO3 can poison the platinum catalyst, platinum could catalyze the oxidation of H3PO3 back to H3PO4.
Experiments Under Realistic Conditions
Using an in-house designed heatable electrochemical cell, compatible with in situ tender X-Ray studies, at the OÆSE end-station recently set up in the Energy Materials In-situ Laboratory Berlin (EMIL), Bär’s team has now analyzed the chemical processes to investigate the oxidation behavior of aqueous H3PO3 under conditions close to HT-PEMFCs working conditions.
They used powerful X-Ray light from the EMIL beamline at the X-Ray source BESSY II, which is available in the tender X-Ray energy range of 2 keV to 5 keV. Oxidation processes from H3PO3 to H3PO4 are monitored in this energy range using X-Ray absorption near-edge structure spectroscopy (XANES) at the P K-edge.
Different Oxidation Reactions Examined
We have thus uncovered different processes for this oxidation reaction, including platinum-catalyzed chemical oxidation, electrochemical oxidation under positive potential bias at the platinum electrode, and heat-promoted oxidation. These in situ spectroscopic results are also confirmed by ion-exchange chromatography and in situ electrochemical characterizations. Remarkably, all of these oxidation pathways involve reactions with water, which shows that the humidity inside the fuel cell has a significant influence on these processes.
Enggar Wibowo, Study First Author and PhD Student, Helmholtz-Zentrum Berlin
Humidity As a Factor for Improvements
The findings suggest that the operating conditions of HT-PEM fuel cells could be improved (for example, by adjusting humidification to oxidize H3PO3 back to H3PO4).
“Corresponding adjustments to the operation conditions of HT-PEMFCs could be implemented to prevent catalyst poisoning by H3PO3 and enhance the efficiency of those fuel cells,” Wibowo added.
Outlook to BESSY III
The work clarifies a key degradation pathway of fuel cells and is a contribution on the way to an H2-based energy supply. It also shows the great benefit of tender X-Rays, and we are looking forward to BESSY III, which aims to close the “tender X-Ray” gap.
Dr. Marcus Bär, Professor, Helmholtz-Zentrum Berlin
Journal Reference:
Wibowo, R. E., et al. (2024) Elucidating the Complex Oxidation Behavior of Aqueous H3PO3 on Pt Electrodes via In Situ Tender X-ray Absorption Near-Edge Structure Spectroscopy at the P K-Edge. Journal of the American Chemical Society. doi:10.1021/jacs.3c12381