Reviewed by Lexie CornerApr 28 2025
A research team, led by Prof. Ya Yan from the Shanghai Institute of Ceramics of the Chinese Academy of Sciences and including scientists from Huazhong University of Science and Technology, Shanghai Jiao Tong University, and the University of Auckland, has developed a stable and efficient catalyst for water oxidation. This development marks progress in green hydrogen production through water splitting technology.
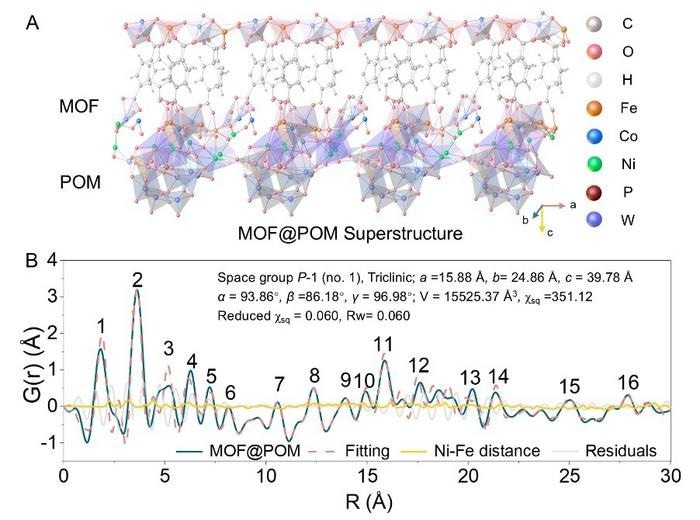 MOF@POM Superstructure and Characterization. Image Credit: Ya Yan
MOF@POM Superstructure and Characterization. Image Credit: Ya Yan
Water oxidation, the process of splitting water molecules into oxygen gas, protons, and electrons, is a critical and rate-limiting step in electrolytic water splitting. This reaction requires significant energy input and proceeds slowly, necessitating effective catalysts to address these challenges.
While existing catalysts based on transition metals demonstrate good activity for water oxidation in alkaline conditions, they often degrade rapidly under the high current densities used in industrial applications. This degradation is mainly caused by structural damage and the loss of active metal sites under strong oxidizing conditions.
To overcome this issue, the researchers proposed a strategy aimed at achieving both high catalytic activity and durability under industrial-level current densities. They accomplished this by grafting cobalt-iron metal-organic frameworks (CoFe-MOF) onto nickel-bridged polyoxometalates (POMs), creating a MOF@POM superstructure.
During water oxidation, the CoFe-MOF underwent an in-situ transformation into a single-layer cobalt-iron layered double hydroxide (CoFe-LDH), which was covalently bonded to POM units via Ni–O bridges. This process resulted in the creation of a highly active and stable single-layer CoFe hydroxide superstructure.
In-situ electrochemical spectroscopy revealed a cooperative catalytic mechanism involving both cobalt and iron active sites, as well as nickel and tungsten tuning centers. The oxidation states of the catalytically active cobalt and iron increased progressively during operation, while the Ni–O and W–O tuning components exhibited dynamic changes in their oxidation states.
The analysis demonstrated that the POM units play a key role in stabilizing the catalyst by influencing electron density and reducing lattice strain. This results in a synergistic strain–electron dual stabilization mechanism, which helps maintain the catalyst's integrity under extreme conditions.
The CoFe-LDH@POM catalyst exhibited high performance in alkaline electrolytes, requiring an overpotential of only 178 mV to achieve a current density of 10 mA/cm², outperforming conventional transition metal-based catalysts. When integrated into an anion exchange membrane electrolyzer, the device achieved a current density of 3 A/cm² at a cell voltage of just 1.78 V at 80 °C, exceeding the US Department of Energy's 2025 industrial target.
Long-term stability tests demonstrated the system's robustness. The electrolyzer operated stably for over 5,140 hours at 2 A/cm² at room temperature, with a minimal voltage decay rate of 0.02 mV/h. At an elevated temperature of 60 °C, the system continued to operate for over 2,000 hours.
This research sets a new benchmark for water oxidation catalysts. It offers a design principle for developing next-generation electrocatalysts, facilitating the potential for industrial-scale, high-current, low-energy alkaline water electrolysis.
Journal Reference:
Yue, K., et al. (2025) Polyoxometalated metal-organic framework superstructure for stable water oxidation. Science. doi.org/10.1126/science.ads1466