Nanalysis benchtop NMR spectrometers are compact, portable and all-in-one instruments. A single enclosure, with a footprint small enough to fit into the large port of a glovebox (11.8 x 11.0 x 19.2”), there is a 1.4 T, 60 MHz hybrid Halbach NdFeB permanent magnet, all necessary electronics, and a touchscreen based interface.
The software is intuitive for those that are familiar with collecting NMR spectra, and easy-to-learn for beginners with easily laid out data acquisition and processing screens. A variety of 1D, 2D or relaxation experiments can be easily loaded and the user can choose to collect data with the pre-set standard parameters or set the desired acquisition parameters through easy drop down and customizable menus. The files can be manipulated onboard or easily exported in standard JCAMP-DX format and processed on most third party NMR processing software tools.
Advantages of Nanalysis 60 in Academic Teaching
In addition to being easy-to-use and affordable, the Nanalysis 60 spectrometers are robust, with no moving parts and no cryogens necessary, there is no preventative maintenance required. Upkeep costs are low, as the instrument uses standard, reusable economy 5 mm NMR tubes, and has minimal power consumption. Whether you are conducting an organic, inorganic, analytical or physical chemistry lab, the Nanalysis 60e is an ideal tool.
Example Undergraduate Experiment
NMR spectroscopy, as it is typically discussed, or at least how it initially taught, tends to refer to static systems. Chemical shifts, integrations and multiplicities are typically reported on fixed structures. For example, pyridine will have a characteristic 1H NMR spectrum in D2O. There are, however, external factors (e.g., concentration, temperature, pH) that change the observed chemical shift. For the case of pyridine, the tricoordinate nitrogen center acts as a Lewis base and is easily protonated in an acidic solution to afford pyridinium. These differences manifest in the observed chemical shifts. 1H NMR Spectroscopy can be used to define a simple acid-base equilibrium equation.
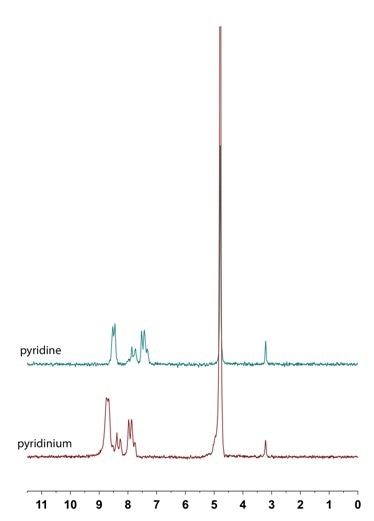
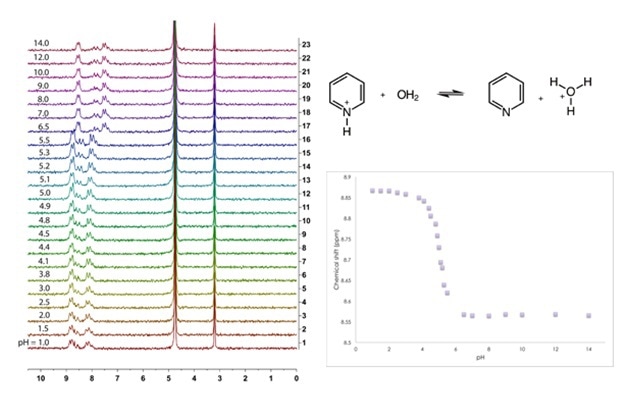
In the intermediate pH range, on the other hand, one would expect that both pyridine and pyridinium to be present in solution, and that this conjugate acid-base pair would be involved in a rapid equilibrium exchange. This can be described by an acid dissociation constant (Ka) expression [1] and arranged to afford the Henderson-Hasselbalch equation [2]. What we observe at intermediate pH then is not the unique chemical shifts of either compound, but rather a weighted, time-averaged chemical shift [3] of the two. This means that the observed chemical shift is a function dependent on: (i) the amount of each species present at a specific pH; and (ii) the chemical shift of each species.
INPUT EQUATIONS
By preparing a pyridine solution, varying the solution pH and observing the effect on chemical shift, one can construct a titration curve – the equivalence point of which is where pKa is equivalent to pH.
Conclusions
The Nanalysis 60 is a teaching tool with many potential applications. Although it can most obviously be used as a supporting tool for synthetic organic labs, it is versatile enough for many multidisciplinary labs and a research support tool. The experiment highlighted here can be viewed as an analytical or physical chemistry experiment, introducing students to the idea of using molecules as pH probes. Although we chose pyridine, it can be done with many nitrogen substrates (e.g., picoline, lutidiene, imidazole). These are often used in biological research to ensure that reaction conditions are consistent between reports and that sensitive analytes do not decompose with differing pH. For more information please visit our website: www.nanalysis.com.

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.