Dec 3 2009
Single-walled nanotubes-cylinders of carbon about a nanometer in diameter-have been highly touted for potential applications such as ultrastrong fibers, electrical wires in molecular devices, or hydrogen storage components for fuel cells. Thanks to a new development by researchers at the National Institute of Standards and Technology (NIST) and five partners, you can add one more application to the list: detection and destruction of an aggressive form of breast cancer.
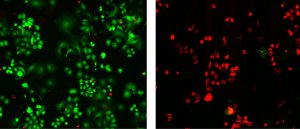 Photomicrographs demonstrate the dramatic impact of using nanotubes to selectively locate and destroy HER2 breast cancer tumors. Tumor cells on the left were treated only with antibodies against the HER2 protein and then irradiated with near-infrared light. Those on the right were treated with a complex of antibodies and nanotubes and then irradiated. Both cultures then were stained with fluorescent dye—green color indicates live cells while red marks areas where cells have been killed. Credit: NIST
Photomicrographs demonstrate the dramatic impact of using nanotubes to selectively locate and destroy HER2 breast cancer tumors. Tumor cells on the left were treated only with antibodies against the HER2 protein and then irradiated with near-infrared light. Those on the right were treated with a complex of antibodies and nanotubes and then irradiated. Both cultures then were stained with fluorescent dye—green color indicates live cells while red marks areas where cells have been killed. Credit: NIST
HER2 is one of a family of genes that help regulate the growth and proliferation of human cells. Normal cells have two copies of HER2, but about 20 to 25 percent of breast cancer cells have multiple copies of the gene, resulting in the overproduction of a HER2-encoded protein (called HER2 and designated in Roman type versus italics for the gene) that is associated with particularly fast growing and difficult to treat tumors. About 40,000 women in the United States are diagnosed annually with this form of breast cancer.
In a recently published paper in BMC Cancer,* the NIST-led research team bonded an antibody that has been created to attack the HER2 protein, chicken immunoglobulin Y (IgY), to short nanotubes (about 90 nanometers long, or 5,000 times shorter than an amoeba). Both halves of the special combination-the antibody and the nanotube-have critical roles to play in selectively hunting down the HER2 tumor cells and eliminating them.
First, the broad genetic differences between avian and human species means that the chicken IgY antibody to HER2 reacts strongly with the target protein expressed on tumor cells while ignoring normal cells with other human proteins. The carbon nanotubes attached to the antibodies also become linked to the HER2 tumors.
Two unique optical properties of carbon nanotubes allow this link to be exploited for improved detection and destruction of HER2 breast cancer cells. Near-infrared laser light at a wavelength of 785 nanometers reflects intensely off the nanotubes, and this strong signal is easily detected by a technique called Raman spectroscopy. Increase the laser light's wavelength to 808, nanometers and it will be absorbed by the nanotubes, incinerating them and anything to which they're attached-in this case, the HER2 tumor cells.
The experiment described in the BMC Cancer paper was conducted in laboratory cell cultures. Using the HER2 IgY-nanotube complex to selectively identify and target HER2 tumors resulted in a nearly 100 percent eradication of the cancer cells while nearby normal cells remained unharmed. In comparison, there only was a slight reduction in cancer cells for cultures treated with anti-HER2 antibody alone.
The next step for the research team is to conduct mouse trials of the HER2 IgY-nanotube complex to see if the dramatic cancer-killing ability works in animals as well as it does in the lab. In a separate but related project, the team hopes to use a nanotube-antibody combination against another tumor cell protein, MUC4, to treat pancreatic cancer.
The research was funded under an interagency agreement between NIST and the National Cancer Institute (NCI), and in part by a grant from the National Science Foundation. Along with scientists from NIST, the research team included members from Rutgers University, Cornell University, the New Jersey Institute of Technology, NCI and Translabion, a private company located in Clarksburg, Md.
* Y. Xiao, X. Gao, O. Taratula, S. Treado, A. Urbas, R.D. Holbrook, R.E. Cavicchi, C.T. Avedisian, S. Mitra, R. Savla, P.D. Wagner, S. Srivastava and H. He. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer, Vol. 9, No. 351, published online Oct. 2, 2009.