Jul 5 2010
Biomerix Corporation announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) Clearance to market its Biomerix Ventral Hernia
Repair Mesh, the latest addition to its innovative portfolio of soft tissue repair devices. The Biomerix Ventral Hernia Repair Mesh, with two distinctly different sides, is the first and only device featuring the Biomerix Biomaterial™ on one side, designed to promote tissue ingrowth, and a resorbable protective film on the other side, designed to minimize tissue attachment to the device in case of direct contact with the viscera. It is indicated for use in hernias and soft tissue deficiencies and for the temporary bridging of fascial defects.
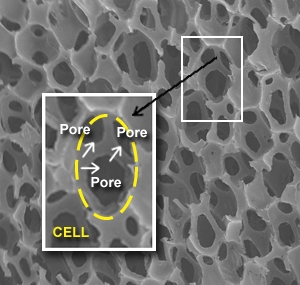 Biomerix Biomaterial™
Biomerix Biomaterial™
More than 400,000 people suffer from ventral hernias in the U.S. today. A ventral hernia is a lump in the abdomen, usually a tissue bulge or a small area of swelling. Ventral hernia is also referred to as an incisional hernia, since it can occur in the area of any prior surgical incision. An aging population and a growing number of obese patients are contributing to the rising incidence of ventral hernias. Current product solutions are unable to address the high recurrence rates and the increased risk of adhesion formation commonly associated with ventral hernia repair. The Biomerix Ventral Hernia Repair Mesh is constructed to support tissue ingrowth. The state-of-the-art design provides a resorbable protective film on one side to act as barrier, minimizing the risk of adhesions to the device. By incorporating the Biomerix Biomaterial™ platform, the Ventral Hernia Repair Mesh's soft and pliable design offers surgeons conformability for acute performance in both open and laparoscopic procedures.
"The FDA clearance of the Biomerix Ventral Hernia Repair Mesh represents the next generation in ventral and incisional hernia repair prosthesis. We believe the novel Biomerix Biomaterial has desirable scaffold features leading to successful repair outcomes, making it a compelling alternative to other synthetic biomaterials," stated Kenneth G. Hayes, President and Chief Executive Officer of Biomerix Corporation.