Feb 1 2011
Researchers in solar energy speak of a day when millions of otherwise fallow square meters of sun-drenched roofs, windows, deserts and even clothing will be integrated with inexpensive solar cells that are many times thinner and lighter than the bulky rooftop panels familiar today.
So, when your iPod is on the nod, you might plug it into your shirt to recharge. Lost in the Serengeti with a sapped cell phone? No problem; rolled in your backpack is a lightweight solar pad. Sailing the seven seas and your GPS needs some juice? Hoist a solar sail and be one with the gods of geosynchronous orbit.
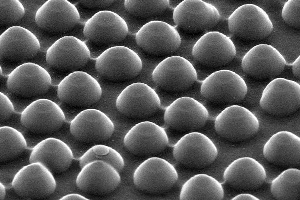 Acting like a waffle iron, silicon nanodomes, each about 300 nanometers in diameter and 200 nanometers tall, imprint a honeycomb pattern of nanoscale dimples into a layer of metal within the solar cell.
Acting like a waffle iron, silicon nanodomes, each about 300 nanometers in diameter and 200 nanometers tall, imprint a honeycomb pattern of nanoscale dimples into a layer of metal within the solar cell.
It is not hard to envision a time when such technologies will be ubiquitous in our increasingly energy-hungry lives. That day may come a bit sooner thanks to a multidisciplinary team of Stanford engineers led by Mike McGehee, Yi Cui and Mark Brongersma, and joined by Michael Graetzel at the École Polytechnique Fédérale de Lausanne (EPFL).
Waves of energy
In an article published in Advance Energy Materials, the Stanford/EPFL team announced a new type of thin solar cell that could offer a new direction for the field. They succeeded in harnessing plasmonics – an emerging branch of science and technology – to more effectively trap light within thin solar cells to improve performance and push them one step closer to daily reality.
"Plasmonics makes it much easier to improve the efficiency of solar cells," said McGehee, an associate professor of materials science and engineering at Stanford.
McGehee is the director of CAMP – the Center for Advanced Molecular Photovoltaics – a multidisciplinary, multi-university team tackling the challenges of thin-film solar cells.
"Using plasmonics we can absorb the light in thinner films than ever before," McGehee said. "The thinner the film, the closer the charged particles are to the electrodes. In essence, more electrons can make it to the electrode to become electricity."
Plasmonics is the study of the interaction of light and metal. Under precise circumstances, these interactions create a flow of high-frequency, dense electrical waves rather than electron particles. The electronic pulse travels in extremely fast waves of greater and lesser density, like sound through the air.
A perfect solar waffle
The lightbulb moment for the team came when they imprinted a honeycomb pattern of nanoscale dimples into a layer of metal within the solar cell. Think of it as a nanoscale waffle, only the bumps on the waffle iron are domes rather than cubes – nanodomes to be exact, each only a few billionths of a meter across.
To fashion their waffle, McGehee and team members spread a thin layer of batter on a transparent, electrically conductive base. This batter is mostly titania, a semi-porous metal that is also transparent to light. Next, they use their nano waffle iron to imprint the dimples into the batter. Next, they layer on some butter – a light-sensitive dye – which oozes into the dimples and pores of the waffle. Lastly, the engineers add some syrup – a layer of silver, which hardens almost immediately.
When all those nanodimples fill up, the result is a pattern of nanodomes on the light-ward side of the silver.
This bumpy layer of silver has two primary benefits. First, it acts as a mirror, scattering unabsorbed light back into the dye for another shot at collection. Second, the light interacts with the silver nanodomes to produce plasmonic effects. Those domes of silver are crucial. Reflectors without them will not produce the desired effect. And any old nanodomes won't do either; they must be just the right diameter and height, and spaced just so, to fully optimize the plasmonics.
If you imagine your nanoself observing one of these solar cells in slow motion, you would see photons enter and pass through the transparent base and the titania (the waffle), at which point some photons would be absorbed by the light-sensitive dye (the butter), creating an electric current. Most of the remaining photons would hit the silver back reflector (the hardened syrup) and bounce back into the solar cell. A certain portion of the photons that reach the silver, however, will strike the nanodomes and cause plasmonic waves to course outward. And there you have it – the first-ever plasmonic dye-sensitized solar cell.
Trapping the light fantastic
It is easy to see why researchers are focused on thin-film solar technology. In recent years, much hope has been directed toward these lightweight, flexible cells that use photosensitive dyes to generate electricity. These cells have many advantages: They are less energy intensive and less costly to produce, flowing like newsprint off huge roll presses. They are thinner even than other "thin" solar cells. They are also printable on flexible bases that can be rolled up and taken virtually anywhere. Many use non-toxic, abundantly available materials, as well – a huge plus in the push for sustainability.
Dye-sensitized solar cells are not without challenges, however. First off, the very best convert only a small percentage of light into electricity – about 8 percent. The bulkier commercial technologies available today have reached 25 percent efficiency, and certain advanced applications have topped 40 percent. And then there is durability. The latest thin solar cell will last about seven years under continuous exposure to the elements. Not bad until you consider that 20 to 30 years is the commercial standard.
Both efficiency and reliability will have to improve. Nonetheless, engineers like McGehee believe that if they can convert just 15 percent of the light into electricity – a figure that is not out of reach – and tease the lifespan to a decade, we might soon find ourselves in the age of personal solar cells. An advance like plasmonics just might provide the spark necessary to take the field down a new and exciting path.
A matter of economics
Cheaper and cleaner will be the keys. Coal-based power is plentiful and cheap, but also comes at a steep environmental cost in gouged landscapes and polluted skies. At today's commercial rates, however, even the best solar alternatives cost five times more per kilowatt-hour than coal. It is clear that economics, and not technology, is what stands between us and our solar future.
But McGehee and others are confident they can make thin solar cells more attractive.