Atoms in metallic glass when squeezed under high pressure form a single crystal. They line up in a regular pattern and in a single direction, while atoms in normal glass lack this order.
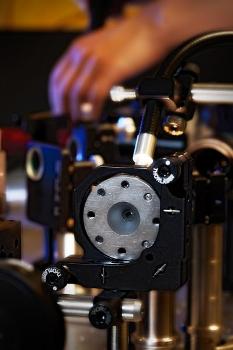 Scientists recently used a diamond anvil like this one to squeeze tiny samples of metallic glass. Under very high pressure, the samples switched from their amorphous, glassy state to form a single crystal -- the first time this behavior has been seen in a glass.
Scientists recently used a diamond anvil like this one to squeeze tiny samples of metallic glass. Under very high pressure, the samples switched from their amorphous, glassy state to form a single crystal -- the first time this behavior has been seen in a glass.
Researchers at the Department of Energy's SLAC National Accelerator Laboratory and Stanford University have discovered this hidden property in glass. The crystalline structure could explain the reason behind the toughness of glass. If each glass piece is a single crystal, then it would not have any weak spots that exist at the crystal boundaries. The discovery will help better understand the behavior and atomic structure of metallic glasses and may help develop new and effective metallic glasses.
Metallic glasses are alloys made of aluminum and cerium. They have useful magnetic properties and are corrosion and wear resistant. They are used in anti-theft tags and power transformers.
The researchers performed the experiment at the Advanced Photon Source facility in Argonne National Laboratory. Applying a high pressure of 250,000 bars, they squeezed the metallic glass samples between two diamond tips. They were, in fact, conducting experiments to assess the behavior of materials under extreme conditions. Due to the applied pressure, the metallic glass samples switched from their glassy state and formed a face-centered cubic crystal.
The high-pressure method provides better insight about the materials' atomic structures by directly connecting highly disorganized glass to highly ordered single crystals. The experiment also paves way for a new method to produce single-crystal materials from glasses.