A research study at the University of Virginia has revealed the details of a novel catalytic site, on which oxidation catalysis taken place.
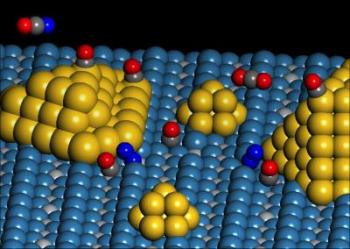 Dual Catalytic Site
Dual Catalytic Site
The research team includes Matthew Neurock, who serves as chemical engineering professor at the School of Engineering and Applied Science, and John Yates, a chemistry professor at the College and Graduate School of Arts & Sciences. Yates stated that the findings pave the way to understand the process of catalysis in an extensive range of materials, as oxidation catalysis is important to several technological applications.
Yates further said that they have both theoretical tools, for instance computational chemistry and experimental tools, for instance spectrometers to investigate catalysis down to the atomic level. The findings could help to design catalysts for all types of catalytic reactions, he added.
The researchers used a titanium dioxide substrate containing nanoscale gold particles for the detection of a special site that functions as a catalyst at the gold and titanium dioxide substrate’s perimeter. During the study, the scientists observed unique molecular transformations and their sites of occurrences on the catalyst. They explained that the major catalytic activity happened on special sites generated at the perimeter area between the titania support and gold particles.
Yates stated that the unique site is named as a dual catalytic site due the presence of different atoms. The researchers observed that an oxygen molecule chemically attaches to the gold atom at the perimeter of the gold cluster and the adjacent titanium atom on the titania support and enters into reaction with an adsorbed carbon monoxide molecule to produce carbon dioxide. They were able to track the intake of carbon monoxide at the unique site utilizing spectroscopy.