Researchers at the Department of Energy (DOE)’s Oak Ridge National Laboratory (ORNL) have utilized a novel technology called electrochemical strain microscopy to study the oxygen evolution/reduction reactions occurring in fuel cells, which will open the door for the development of enhanced materials and devices.
 Novel Microscopy Method allows researchers to study key reactions in fuel cells
Novel Microscopy Method allows researchers to study key reactions in fuel cells
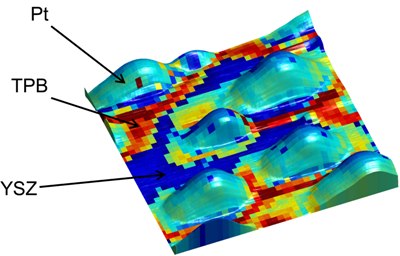
Huge quantities of platinum are utilized as catalysts for the oxygen-reduction reaction that manipulates the efficacy and durability of the fuel cells. However, current electrochemical technologies are not capable of investigating the mechanism and the location of the reaction at the nano-level.
ORNL’s Sergei Kalinin stated that the mobile ions in the fuel cell materials act like a liquid. The materials with quicker mobile ions could serve as better fuel cell materials, he said. Electrochemical strain microscopy was able to capture this ion mobility, he added. Other electrochemical technologies were not able to investigate oxygen-reduction reactions as they are restricted to resolutions at the microscale, which is 10,000 times bigger than a nanoscale.
Kalinin further said there is a vast gap between basic science and applied science for energy systems such as batteries and fuel cells. The energy systems are highly complicated than the semiconductor industry where the link between fundamental science and application is well established, he added.
The research team conducted the study at the Center for Nanophase Materials Sciences (CNMS) at ORNL. CNMS is one among the five DOE Nanoscale Science Research Centers endorsed by the DOE Office of Science.