A new bio-compatible three-dimensional printing material suitable for dental and medical applications has been introduced by Objet, one of the leading providers of high quality and cost- effective inkjet-based three-dimensional printing materials and systems.
 Bio-Compatible 3D printing material
Bio-Compatible 3D printing material
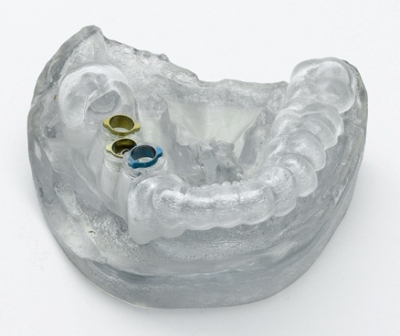
MED610 material integrates bio-compatibility with clear transparency and high dimensional stability. These enhanced features deliver benefits to the whole dental and medical workflow starting from surgical planning to the development of the procedure itself. The bio-compatible material can be utilized on all Objet Connex and Eden lines of three-dimensional printers and is available for immediate purchase.
MED610 is used for polymethylmethacrylate (PMMA) simulation and also for the production of customized, precise surgical guides. The enhanced material transparency provides better examination of soft tissues during medical and dental procedures. Objet’s MED610 printing material is suitable for extended mucosal-membrane contact of about 24 hours and skin contact of more than 30 days.
The material has been evaluated for biocompatibility in accordance with standard ISO 10993-1 and has received five medical approvals related to USP Plastic Class VI, irritation, delayed type hypersensitivity, genotoxicity and cytotoxicity. It has also received ISO 13485:2003 certification for bio-compatibility. MED610 becomes part of Objet VeroDent material that is currently used in the digital dental process by global dental laboratories.