A research team from the Brookhaven National Laboratory of the U.S. Department of Energy has created a new electrocatalyst using low-cost materials for the production of clean hydrogen gas from water.
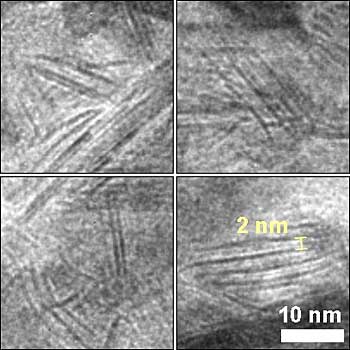 This magnified image from a transmission electron microscope reveals details of the unexpected nanosheet structure of the nickel-molybdenum-nitride catalyst, seen here as dark, straight lines.
This magnified image from a transmission electron microscope reveals details of the unexpected nanosheet structure of the nickel-molybdenum-nitride catalyst, seen here as dark, straight lines.
The research team’s quest for an affordable, high-activity catalyst resulted in the development of the nickel-molybdenum-nitride catalyst. What surprised the team was this Goldilocks compound’s high-performing nanosheet structure, which provides a new form for efficient hydrogen catalysis.
In this novel electrocatalyst, nickel plays the catalytic role of platinum, the gold standard for electrocatalysis. However, nickel required an electron density comparable to that of platinum. Hence, the team added metallic molybdenum to increase the reactivity of nickel. Nevertheless, the performance level of nickel was still lower than that of platinum.
Therefore, the team treated the nickel-molybdenum compound under a high-temperature ammonia environment, which not only caused the compound to get infused with nitrogen but also converted the particles into 2D nanosheets, which have high reactivity because have highly accessible reactive sites.
Wei-Fu Chen explained that the addition of nitrogen increased the nickel-molybdenum’s electron density, expanded its lattice, prevented corrosion, and created an electronic structure close to that of noble metals.
Project leader, James Muckerman stated that the performance of the new electrocatalyst is close to that of platinum but it has achieved an unprecedented electrocatalytic activity and stability when compared to other non-noble metal compounds. Since is the production process is easy and scalable, the nickel-molybdenum-nitride catalyst is suitable for a broad range of industrial applications. Although this catalyst is not a comprehensive solution to produce low-cost hydrogen gas, it is helpful in lowering the cost of key equipment.