Oct 11 2012
Despite their low natural abundance, rare sugars have enormous potential in several important applications, including their use as components for antiviral drugs, low-calorie sweeteners with low glycemic indexes, anti-inflammatory agents with immunosuppressive properties and chiral building blocks in natural products synthesis.
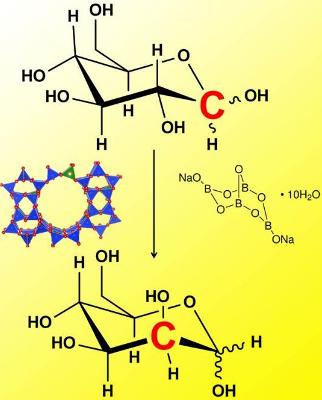 The process of epimerization with Sn-Beta zeolites with borate salts. Image courtesy: Roman Lab
The process of epimerization with Sn-Beta zeolites with borate salts. Image courtesy: Roman Lab
In order to meet today’s high demand for the sugars, scientists currently use costly and complex biochemical processes to transform easily found abundant sugars into these rare ones.
These biochemical processes use three main classes of enzymes, two of which — isomerases and oxidoreductases — are popular because they are active on a wide range of simple substrates; however such general activity is not always an advantage because it can result in the formation of side products (for example xylose isomerase converts glucose into fructose and simultaneously converts fructose into mannose). These processes have generated some commercially available rare sugars, but the complex nature of that biochemical process makes it very costly. The third class of enzymes, epimerases, are potentially the most useful biocatalysts for the widespread production of rare sugars because they offer high specificity for products and are capable of selectively modifying sugars at multiple carbon positions. But like all biological catalysts, epimerases can be fragile because they require very specific reaction conditions and can only act on previously functionalized sugars.
In a paper published this week in Nature Communications, an MIT team has found a way to use inorganic catalysts in place of the enzymes to generate sugar epimers in a simple and robust manner. The catalytic system involves using a tin-substituted microporous silicate (Sn-Beta zeolite) combined with a borate salt. "This work is exciting because the zeolite/borate combination generates sugar epimers with yields similar to those typically observed with biological catalysts,” explains Professor Yuriy Román, Texaco-Mangelsdorf Assistant Professor of Chemical Engineering, “Inorganic materials can rarely perform as well as their biological counterparts in the selective conversion of sugars."
MIT graduate student and lead author William R. Gunther said, "Our system opens up exciting opportunities for the production of rare sugars using inorganic catalysts, such as L-ribose, which can be used in anti-viral and anti-cancer agents.”
Román and his team found that Sn-Beta zeolite in the presence of sodium tetraborate is shown to catalyze the selective epimerization of aldoses in aqueous media. The reaction proceeds by way of a rather unusual 1,2 carbon-shift mechanism, wherein C-C bonds move within the molecule’s backbone.