Jun 3 2014
Landmark study opens doors to further studies into chemical modification of materials for alternative energy conversion
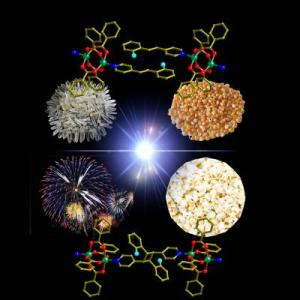 This is a schematic diagram showing the popping nature of the crystals under UV light, a property that is very similar to the popping of corns on a hot plate. Credit: National University of Singapore
This is a schematic diagram showing the popping nature of the crystals under UV light, a property that is very similar to the popping of corns on a hot plate. Credit: National University of Singapore
A team of international scientists led by Professor Jagadese J Vittal of the Department of Chemistry at the National University of Singapore's (NUS) Faculty of Science has successfully unraveled the chemical reaction responsible for propelling microscopic crystals to leap distances up to hundreds of times their own size when they are exposed to ultraviolet (UV) light.
This popping effect, akin to the bursting of popcorn kernels at high temperatures, demonstrates the conversion of light into mechanical motion. It is the first instance of a "photosalient effect" driven by a photochemical reaction in solids to be reported. The rare phenomenon provides a new way to transfer light energy into mechanical motion, and potentially offers a fresh approach to harness solar energy to power light-driven actuators and mechanical devices.
These novel findings were published as the cover story in the English version of German scientific journal Angewandte Chemie International Edition on 2 June 2014.
Popcorn-like explosion of tiny crystals demonstrated
The NUS team has been actively looking for ways to control the reactivity of solids. While studying the metal complex polymerisation in the solid state, Mr Raghavender Medishetty, a PhD candidate, and Ms Bai Zhaozhi, a third-year undergraduate student, of the Department of Chemistry at the NUS Faculty of Science, found that very tiny crystals leap violently when exposed to UV light. Interestingly, even when the crystals are irradiated with weak UV light, the single crystals burst violently to travel up to hundreds of times their sizes. Such a distance is equivalent to that of a human jumping few hundred metres.
To understand the reactions behind the self-actuation of the crystals, the NUS team worked with a research team from the New York University Abu Dhabi led by Associate Professor Panče Naumov to capture the rapid motion of the crystals with an optical microscope coupled to a high-speed camera. They also collaborated with a research team from the Max Plank Institute for Solid State Research in Germany, led by Professor Robert E. Dinnebier to model the kinetics by time-resolved powder X-ray diffraction methods.
Through the use of a variety of analytical methods, the researchers discovered that the cause for the popping and disintegration of these single crystals was due to the strain generated during the photochemical reaction in the crystal, leading to the formation of metal coordination polymers. Sudden expansion of volume during this reaction results in the release of the stress in the form of ballistic events. Such a chemical reaction is very similar to the popping of corn kernels on a hot plate as a result of rapid expansion of the inner kernel compared to the outer shell.
Elaborating on the findings, Prof Vittal said, "Photoactuated movements are induced by the application of light to certain type of crystals, but they are observed to be less efficient than the biomechanical motions of plant and animal tissues. In our work, we observed that the conversion of energy in the crystals may be able to mimic the motility of biological systems and provide a new way to transfer light energy into mechanical motion."
He added, "Our work validates that the so called "bad" UV light from sources such as the sun can be utilised to convert chemical reactions to drive mechanical motions with practical uses. Knowledge and application of such behaviour is very important towards addressing the global energy crisis."
This study opens doors for further studies into materials for alternative energy conversion.
Further research
The NUS research team is examining a series of new compounds to better understand the mechanism and enhance the efficiency of the photosalient effect. They are also conducting systematic studies to look into the effects of chemical modification on the photosalient effect.
The team hopes to eventually develop new materials that could convert solar energy effectively into mechanical energy. In addition, the team also hopes to leverage on the principle of the photosalient effect to create a new source of reversible chemical energy by controlling the shape and size of crystals used for energy conversion.