Aug 5 2014
In a paper published this week in the journal Nature Chemistry, researchers from the Center for Electrochemical Sciences – CES at the Ruhr-University Bochum and from the Max-Planck-Institute for Chemical Energy Conversion in Mülheim an der Ruhr report a novel concept to work with efficient and possibly cheaper catalysts.
A kind of buffer protects the catalysts against the hostile conditions encountered in fuel cells, which have been to date dismissed utilization. The scientists report in the current issue of Nature Chemistry.
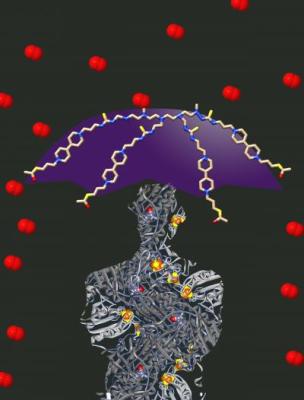 Redox hydrogel works like a protective umbrella against oxygen. Credit: RESOLV
Redox hydrogel works like a protective umbrella against oxygen. Credit: RESOLV
Hydrogenases, an alternative to platinum?
Bio-catalysts for hydrogen oxidation – so called hydrogenases – are also part of nature. They developed just by using elements available to living organisms, that means without any noble metals. Complex hydrogenases evolved based solely on abundant elements such as nickel and iron. The most efficient hydrogenases reach the turnover rate of platinum and the supply of the elements they consist of is virtually unlimited. "For this reason Hydrogenases are potentially interesting alternatives to noble metals", says Prof. Dr. Wolfgang Schuhmann (Professorship for analytical chemistry at the RUB). Though hydrogenases are not able to work while being under the constraints encountered in a fuel cell. Traces of oxygen and extreme electrical potentials cause deactivation processes.
Redox hydrogel, a shield for efficient but sensitive catalysts
The team in Bochum and Mülheim focused nevertheless on a new strategy to accommodate sensitive catalyst to the working conditions of standard fuel cells. The key idea is to shield the catalyst in a protective matrix with properties specifically designed to prevent the deactivation processes. Instead of contacting the hydrogenase directly to the electrode, an immobilization in a redox hydrogel shall protect the construct. It is designed to serve both as a redox buffer and an oxygen scavenger. Hence, within the hydrogel film, neither high potential, nor oxygen affects the bio-catalyst. Under working conditions the hydrogel-modified fuel cell is able to convert chemical energy from hydrogen into electrical energy over several weeks, while in absence of the hydrogel, the hydrogenase is deactivated within seconds.
A major step toward a novel fuel cell redesign
"The hydrogel concept opens the possibility to integrate a variety of other sensitive biological or artificial catalyst for which the intrinsic stability cannot be further increased", says Prof. Wolfgang Lubitz, Director at the MPI CEC. "This is a major step toward a novel fuel cell redesign, which may reposition them at the forefront in the race toward global sustainable energy industry."
This news release is available in German.