Ghent University researchers have discovered a method for reversibly converting a thermoset polymer into a thermoplastic, using "click chemistry" based on chemicals called triazolinediones (TADs).
 This image shows bead of water sitting on top of water resistant porous coordination polymer crystals. Credit: Kyoto University iCeMS Public Relations
This image shows bead of water sitting on top of water resistant porous coordination polymer crystals. Credit: Kyoto University iCeMS Public Relations
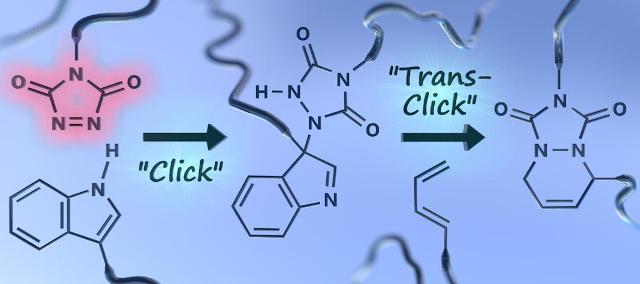
In general, the TAD molecules are stable and unreactive. However, they readily react with the electron-rich molecules of unsaturated organic compounds, such as polyurethane. This property means they can be used to cross-link the polymer chains, forming a thermosetting polymer with macromolecular crosslinks.
The crosslinking reaction can take place under ambient conditions, and are insensitive to moisture and air, without the help of catalyst. The main benefit of the TAD crosslinkers, however, is their reversiblity - the polymer can be unlinked at temperatures of 120°C, if the reaction partner includes an indole group. This allows the thermoset material to be re-cast or extruded, whilst maintaining the properties of a thermoset polymer during use.
TAD reagents have excellent reaction kinetics and relatively low energy barriers in terms of breaking and formation of reaction bonds. The researchers explained that this is the key to enabling the simple "click" reaction at room temperature, whilst also allowing for the reverse "unclick" reaction to take place at attainable temperatures.
As an added bonus, the un-reacted TAD molecules appear bright red in color - this color disappears during the click reaction, providing instant visual feedback for the user.
This research was published in the journal Nature Chemistry.