Oct 31 2015
Designing alloys to withstand extreme environments is a fundamental challenge for materials scientists. Energy from radiation can create imperfections in alloys, so researchers in an Energy Frontier Research Center led by the Department of Energy's Oak Ridge National Laboratory are investigating ways to design structural materials that develop fewer, smaller flaws under irradiation.
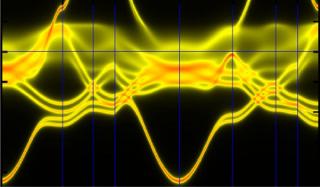 In complex alloys, chemical disorder results from a greater variety of elements than found in traditional alloys. Traces here indicate electronic states in a complex alloy; smeared traces reduced electrical and thermal conductivity. Credit: Image credit: Oak Ridge National Laboratory, U.S. Dept. of Energy. Image by G. Malcolm Stocks
In complex alloys, chemical disorder results from a greater variety of elements than found in traditional alloys. Traces here indicate electronic states in a complex alloy; smeared traces reduced electrical and thermal conductivity. Credit: Image credit: Oak Ridge National Laboratory, U.S. Dept. of Energy. Image by G. Malcolm Stocks
The key, they report in the journal Nature Communications, is exploiting the complexity that is present when alloys are made with equal amounts of up to four different metallic elements.
"Chemical complexity gives us a way to modify paths for energy dissipation and defect evolution," said first author Yanwen Zhang, who directs an Energy Frontier Research Center, called "Energy Dissipation to Defect Evolution," or "EDDE," funded by the U.S. Department of Energy Office of Science. The growing center is nearly 15 months old and brings together more than two dozen researchers with experimental and modeling expertise. EDDE has partners at Oak Ridge, Los Alamos and Lawrence Livermore national laboratories and the universities of Michigan, Wisconsin-Madison and Tennessee-Knoxville.
Radiation can harm spacecraft, nuclear power plants and high-energy accelerators. Nuclear reactions produce energetic particles--ions and neutrons--that can damage materials as their energy disperses, causing the formation of flaws that evolve over time. Advanced structural materials that can withstand radiation are a critical national need for nuclear reactor applications. Today, nuclear reactors provide one-fifth of U.S. electricity. Next-generation reactors will be expected to serve over longer lifetimes and withstand higher irradiation levels.
In a reactor, thousands of atoms can be set in motion by one energetic particle that displaces them from sites in a crystal lattice. While most of the displaced atoms return to lattice sites as the energy is dissipated, some do not. Irradiation can damage structural materials made of well-ordered atoms packed in a lattice--even obliterating its crystallinity. Existing knowledge of radiation effects on structural materials is mostly about reactor-core components. Over the life of a typical light water reactor, all atoms in the structural components can be displaced on average 20 times, and accumulated damage may threaten material performance. To prepare for new reactor concepts, scientists will have to design next-generation nuclear materials to withstand atoms displaced more than 200 times--a true "grand challenge."
Recipes for success
Since the dawn of the Iron Age 3,000 years ago, useful metallic alloys have typically comprised multiple phases with one or two dominate elements modified by additions of other elements. The Gateway Arch in St. Louis, for example, is a conventional stainless steel--iron with additions of chromium and nickel, low concentrations of carbon, and even smaller amounts of manganese, silicon, phosphorus and sulfur. Recently, a very different class of materials has generated a great deal of interest. In these special alloys, several different types of atoms, in equal proportions, distribute randomly in a simple crystal lattice. High entropy alloys comprising five or more species are exemplars. Indeed, researchers at Berkeley and Oak Ridge labs have recently shown that some of the alloys, discovered about a decade ago, exhibit exceptional strength and ductility at cryogenic temperatures. In all these alloys, chemical disorder is intrinsic to their behavior.
In comparison, the goal of the EDDE study was to find how compositional complexity can lead to differences in heat and electricity conduction and influence defect dynamics at early stages that affect the robustness of a structural material at later stages. The results revealed how advanced alloys achieve greatly enhanced irradiation performance through chemical diversity (spoiler alert: they optimize electronic structure and atomic arrangements).
It took a team with many skills to explore a novel set of alloys containing nickel and equal amounts of other elements. One recipe tried duos of ingredients (e.g., nickel-cobalt) whereas others tried triplets (e.g., nickel-chromium-cobalt) or quartets (e.g., nickel-chromium-iron-cobalt). The chemical elements, distributed randomly in the crystal lattice, create unique site-to-site, microscopic distortions. The lattice nonetheless retains its macroscopic crystalline structure.
Integrating theory and experiment, the scientists grew alloy crystals of unrivaled quality, calculated changes to electronic structures and intrinsic transport properties that were induced by chemical disorder, and confirmed the computational results with experimental measurements of each crystal's electrical resistivity and thermal conductivity. The results from ion irradiation, modeling of defect production, ion-beam analysis and microstructural characterization of the irradiated alloys show significantly reduced defect production and damage accumulation in these materials. The findings suggest a link between slow energy dissipation and suppressed defect evolution.
"We observed suppressed damage accumulation with increasing chemical disorder from pure nickel to binary and to more complex quaternary [alloys]," Zhang said.
A material's electronic band structure determines how well electrons can conduct electricity and heat. In a typical metal, say a metallic crystal of pure nickel, energy dissipates quickly because electrons barely scatter--when an energetic particle hits the perfect atomic ordering of the crystal, the resulting energy wave is free of obstructions and can rapidly propagate, leaving little energy at the collision site. In the willy-nilly atomic arrangement of a multicomponent disordered alloy crystal, however, when the energetic particle hits a lattice atom, the energy encounters obstructions and stays local, and for a longer time.
The EDDE study showed that fewer and smaller defects were produced as the alloy complexity increased. It also showed dramatic improvement in properties related to resistance to radiation damage.
It turns out that just increasing the number of elements (and therefore the disorder, or entropy) in the recipe doesn't necessarily produce the best alloys for targeted functions. Determining what combinations work best depends on aspects including local structural distortions and chemical, electronic and magnetic properties of constituent atoms.
With dramatically lower electrical and thermal conductivity than traditional alloys, next-generation alloys based on recipes with high chemical disorder may slow energy dissipation and experience far fewer of the defects that weaken structural materials over time. Evidence that slow energy dissipation can remove some local defects even hints at the possibility of developing self-healing nuclear structural materials.
Further studies are needed to understand how alloy complexity can tailor material properties. The knowledge gained may spur new design principles of alloys for advanced energy systems and reduce trial-and-error to accelerate "materials by design."
"These insights into defect dynamics at the level of atoms and electrons provide an innovative path forward toward solving a long-standing challenge in structural materials," Zhang said.