A novel material that displays both the stiff behavior or a solid and the soft, visoelastic behavior of a polymer gel has been developed. The material, which can be eaily cutomised, is a hybrid of a metal organic cage and a metallogel and could be used for specific gas storage, targeted drug delivery and water filtration.
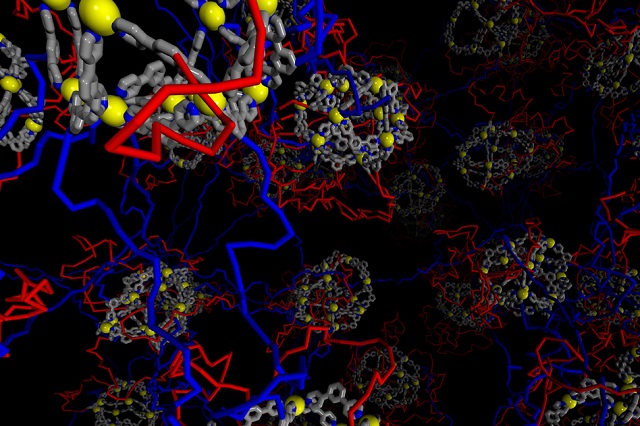 MIT chemists have created a new type of gel by linking metal organic cages with long polymer strands (blue). The red polymer strands, which loop back to the cages, can be used to further customize the gels by adding other molecules. (Image courtesy of the researchers)
MIT chemists have created a new type of gel by linking metal organic cages with long polymer strands (blue). The red polymer strands, which loop back to the cages, can be used to further customize the gels by adding other molecules. (Image courtesy of the researchers)
The novel adaptable metallic-cage gels are called polyMOCs, and show promise for use in gas storage, targeted drug release and water filtration. They are a hybrid material of metal organic cages (MOCs) and metallogels, and their stiff structure is rendered by metal-based clusters. Similar to standard polymer gels metallogels include metals that are adhered to polymer chains, whilst MOCs have a stiff structure and are known to form crystalline materials.
One can imagine a class of materials that borrows from both of those, and so has the well-defined, self-assembled structures of the MOCs, but also has the viscoelastic properties of a polymer gel. That’s what we’ve tried to make.
Prof. Jeremiah Johnson - MIT
The team at MIT, led by Aleksandr Zhukhovitskiy, along with other researchers successfully developed the gels using a metallo-supramolecular assembly technique. Using this novel approach, the team created pyramids, paddlewheels, spheres and other 3D shapes by combining polymers. The polymers were linked together using ligands, which are a class of organic compounds. Ligands can bridge the gaps between individual polymers as they are capable of coordinating, i.e. attaching, to multiple metal atoms on different polymer chains.
For the experiment, a ligand was used that included two pyridine groups, with each group capable of coordinating to the metal palladium. Each palladium atom is can form bonds with four other ligand molecules, this ultimately produces a cage-like structure with 24 ligands and 12 palladium centers.
The centers also couple with other similar metallic cages through viscoelastic polymer links. The overall effect of all of these intramolecular connections is the formation a huge self-assembled gel.
Up to 24 polymer chains are linked to each metal cage, of which four or five polymer chains bond with other metal cages. The remaining unattached polymer chains loop back and link to their own metal cage. Such loops are considered defective.
However, the MIT researchers exploited the defective behavior and added functional molecules in the place of certain ligands which allowed them to develop an optimized material. For their experiment, the team replaced certain looped ligands with pyrene, a fluorescent molecule.
We can take the ligands that aren’t connected to another cage and swap those out, while keeping the same net number of chains connecting junctions,” said Johnson. “This allows us to make completely different materials in terms of their composition, but they can have the same mechanical properties.
Prof. Jeremiah Johnson - MIT
By using these clusters of metallic organic cages, they’ve been able to increase the functionality, and this gives the materials very different properties and mechanical behavior. It’s very elegant, fundamental science that opens the door to a whole range of directions.
Prof. Stuart Rowan - Case Western Reserve University
When we look at this material under a UV light it’s fluorescent, but mechanically it’s identical to a material without the pyrene ligand. The modulus is the same, the swelling behavior is the same, but now this gel is intensely fluorescent.
Prof. Jeremiah Johnson - MIT
Johnson added that different types of molecules with different functions can be added to the new material. Drug molecules could be stored within the metal cages for drug delivery applications. Such gels also hold potential for hydrogen storage to run fuel cell-based cars. The new material could also be tuned for water purification applications through the addition of ligands to capture and isolate heavy metals.
You could imagine attaching all kinds of things onto those extra ligands to adapt the material for applications of interest. Currently we’re working on making ligands that can not only put something outside of the cage, but also inside the cage, so we could do controlled uptake or release of molecules from the inside of these cages.
Prof. Jeremiah Johnson - MIT
Further research is going on to develop similar gels with various cage shapes, and to design materials using potentially less toxic and cheaper metals than palladium; such as titanium, iron, and zinc.
The research has been described in the journal Nature Chemistry.