Feb 9 2016
Rapid freezing of any liquid or even liquid metal leads to the formation of glass. Materials science research is currently focusing on metallic glasses or vitrified metals that have been formed by swiftly cooling alloys of a wide range of metals such as magnesium, copper, titanium, palladium, zirconium, and iron.
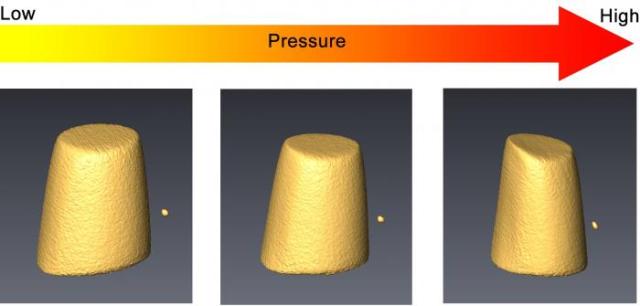 The volume change of cerium-based metallic glass during compression, measured by transmission x-ray microscopy technique in a diamond anvil cell. (Image is provided courtesy of Qiaoshi “Charles” Zeng.)
The volume change of cerium-based metallic glass during compression, measured by transmission x-ray microscopy technique in a diamond anvil cell. (Image is provided courtesy of Qiaoshi “Charles” Zeng.)
Metallic glasses or vitrified metals are used in a wide variety of applications ranging from the manufacture of golf clubs to aerospace construction. Despite such applications, metallic glasses still remain to be poorly understood.
A research team including Ho-kwang “Dave” Mao and Carnegie’s Qiaoshi “Charles” Zeng is currently focusing on identifying rules, which control the creation of metallic glasses. This task is being carried out by analyzing metallic glasses under high pressures. High-pressure research helps understanding a material’s state of disorder or order and also to examine a structure on an atomic level.
Crystals are designed in repeating patterns that stretch in all directions. This kind of a firm pattern is not present in glasses. Crystalline metals existing at the borders between crystal grains very often have their weaknesses.
Practically, metallic glasses are hard, very strong, and resistant to corrosion and wear, all of which help them to be excellent potential candidates for engineering applications such as electronics casings, and medical applications like stents and surgical pins. Their creation is still considered to be a costly and time-intensive process because scientists are yet to develop a general metallic glass theory. It would be a revolutionary outcome if basic rules are developed to assist the development of metallic glasses.
The team, which also included Carnegie’s Zhidan Zeng, Stanislav Sinogeikin, Yoshio Kono, Curtis Kenney-Benson, Changyong Park, and Wenge Yang, examined glass developed from alloys of the metal cerium under pressure. High-pressure research teams at Carnegie have extensively analyzed cerium alloys for this research. Their aim was to identify the basic rules that correlate the properties and structure of metallic glasses to support their further research and synthesis. Their findings have been published in Proceedings of the National Academy of Sciences.
High pressure compression is an extremely powerful tool for fine-tuning both the structures and the properties of materials such as metallic glasses and for looking for information that is consistent across samples. By accurately measuring the evolution of structure and properties during compression, we could establish a relationship between them. The more we learn about the structure-property relationship in metallic glass, the better we can predict the properties of other potential metallic glasses.
Zhidan Zeng, Lead Author
The team, through its study on cerium-based metallic glass, discovered an appropriate rule that develops a precise numeric relationship between the declining volumes in metallic glasses under pressure with regard to its atomic structure, which is determined using improved X-ray tools. This numeric relationship is also applicable for metallic glasses under ambient pressure.
The exactness and universality of this rule we’ve discovered indicates that the atomic structures of metallic glasses are not totally random, even if they aren’t as regularly packed as crystalline metals; they may share some predictable structural features. This is an important step in understanding what controls the formation of these materials.
Zhidan Zeng, Lead Author
This research was supported by the National Natural Science Foundation of China, the NSF, and the DOE.