Mar 25 2016
A decade ago, Donald Sadoway, a MIT professor, and his students developed liquid metal batteries, which now appear to be a potential solution to make renewable energy more affordable and practical. These batteries are capable of storing huge amounts of energy, and can balance the fluctuations of production and consumption of power. Ambri, a Cambridge-based startup company, is now in the process of commercializing these liquid metal batteries.
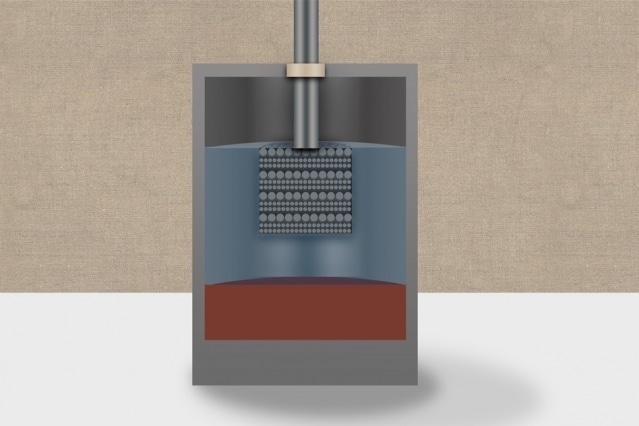 An artist’s rendering of a calcium liquid battery. Illustration: Christine Daniloff/MIT
An artist’s rendering of a calcium liquid battery. Illustration: Christine Daniloff/MIT
Sadoway and his research group have discovered another set of chemical constituents, which could possibly make the technology more affordable and practical. This also presents a range of possible differences that could exploit local resources.
Sadoway, the John F. Elliott Professor of Materials Chemistry, postdoc Takanari Ouchi, Hojong Kim, who is now a professor at Penn State University, and PhD student Brian Spatocco at MIT, have reported the study results in the Nature Communications journal.
The team demonstrated that calcium can form the basis for the molten salt, which forms the central layer of the three-layer battery, as well as the negative electrode layer. Sadoway informed that this was an unexpected finding. Calcium, a low-cost and abundantly available element, exhibits certain properties which made it unsuitable for use in this type of battery.
An important property of the liquid battery is that an individual layer is formed by each of its three constituents depending on the varied densities of materials, just like varied liqueurs isolate in certain novelty cocktails. Calcium, on the other hand, can easily dissolve in salt. It is important to ensure that these layers do not combine at their edges and also sustain their discrete identities.
Ouchi was particularly attracted to this apparent complexity of using calcium in liquid metal batteries. He says “It was the most difficult chemistry” to make it work yet had possible advantages due to the low cost of calcium and due to its intrinsic high electrical energy as a negative electrode. “For me, I’m happiest with whatever is most difficult,” he says — which is a typical attitude at MIT, Sadoway pointed out. The high melting point of calcium was another major issue and would have caused the battery to work at 900oC “which is ridiculous,” Sadoway says.
However, these issues could be resolved. The team first dealt with the temperature issues by alloying the calcium with magnesium, another low-cost metal with a relatively lower melting point. The ensuing combination led to a lower operating temperature, which was approximately 300oC less than that of purified calcium, while simultaneously maintaining the calcium’s high-voltage benefit. The other major advancement made by the research team was that during the development of salt, which is utilized in the battery’s central layer known as electrolyte, that charge carriers (ions) should be able to cross as and when the battery is utilized. The relocation of those charge carriers is followed by an electric charge that passes via the wires linked to the lower and upper molten metal layers, the batteries electrodes.
A combination of calcium chloride and lithium chloride are included in the new salt formulation. It was observed that the alloy of calcium and magnesium does not dissolve adequately in this form of salt, resolving the other issue related to the use of calcium in liquid batteries.
Conversely, resolving that issue resulted in a new finding. An 'itinerant ion' usually travels via the electrolyte in a rechargeable battery, for instance, sodium in sodium-sulfur or lithium in lithium-ion batteries. However, in this situation, it was observed that many ions in the molten-salt electrolyte play a role in the flow, increasing the overall energy output of the battery. This was an unexpected finding, which could provide new opportunities in battery design, Sadoway said.
The researchers also observed another possibility in the novel battery chemistry.
There’s an irony here. If you’re trying to find high-purity ore bodies, magnesium and calcium are often found together.
Donald Sadoway, Professor, MIT
Removing the magnesium contaminant from the calcium or vice versa takes a significant amount of effort and energy. However, given that the material required for the electrode in these batteries is a combination of the two, the initial costs related to the materials can possibly be saved by utilizing lower grades of magnesium and calcium containing some form of the other.
There’s a whole level of supply-chain optimization that people haven’t thought about.
Donald Sadoway, Professor, MIT
According to Ouchi and Sadoway, these specific chemical combinations are just the starting point that could form the basis to develop innovative techniques for battery formulations.
As all of the liquid batteries, such as those that are being developed at Ambri and the original liquid battery materials, would utilize analogous insulating systems, containers, and electronic control systems, the batteries’ exact internal chemistry may evolve over time. The liquid batteries could also adapt to accommodate materials availability and local conditions while still utilizing the same components.
The lesson here is to explore different chemistries and be ready for changing market conditions.
Donald Sadoway, Professor, MIT
Sadoway added that what they have developed is not a battery, but an entire battery field. In due course, individuals could study more components of the periodic table to discover more improved formulations
This paper brings together innovative engineering advances in cell design and component materials within a strategic framework of ‘cost-based discovery’ that is amenable to the massive scale-up required of grid-scale applications. [Since the study is based on a well-developed electrochemical systems employed in the production of aluminum] the path forward to grid-scale applications can therefore take advantage of a large body of existing engineering experience in areas of sustainability, environmental, life cycle, materials, manufacturing cost, and scale-up.
Richard Alkire, Professor of Chemical and Biomolecular Engineering, University of Illinois
The U.S. Department of Energy’s Advanced Research Projects Energy (ARPA-E) and the French energy company Total S.A funded the study.