May 4 2016
A beneficial biofilm with the capability of preventing the biofouling of reverse osmosis (RO) membranes has been developed by a team of chemical engineers from Pennsylvania State University (Penn State).
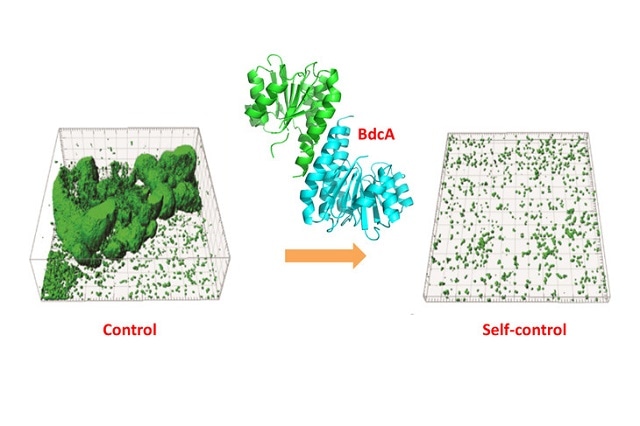 Bioengineered "beneficial biofilms" may prevent the biofouling of reverse osmosis (RO) membranes. (Image Credit: Thomas Wood)
Bioengineered "beneficial biofilms" may prevent the biofouling of reverse osmosis (RO) membranes. (Image Credit: Thomas Wood)
Through a quorum-sensing circuit, the thickness of the membranes can be limited by the biofilm, eventually reducing the chance of biofouling in membrane-based water treatment systems by discharging chemicals that keep undesirable bacteria away.
We realized that the accumulation of microbial films in water treatment membranes is unavoidable. But just like good bacteria exists in your gut to keep you healthy, we predicted that helpful bacteria in RO may be able to prevent the unchecked reproduction of harmful biofilms. Essentially, our method is a ‘probiotic-approach’ to combat biofouling.
Manish Kumar, Assistant Professor of Chemical Engineering, Penn State
The availability of safe and clean water is an increasingly global concern. Membrane filtration technologies are widely considered as a viable option to use readily abundant yet low quality water resources like brackish water, seawater, and recycled wastewater in order to address this demand.
However, these membrane systems have to deal with complications such as the formation of biofouling caused by the accumulation of bacteria and microbes membrane surfaces. This leads to clogging and reduces membrane permeability. As a result, the energy required for the operation of such systems increases as a whole.
To address this issue, Kumar in close cooperation with Thomas Wood, Endowed Biotechnology Chair Professor of Chemical Engineering, and Tammy Wood, research associate in the Department of Chemical Engineering, studied interactions between the membranes and biofilms and their biochemical attributes.
Department of Chemical Engineering graduate student Rajarshi Guha and postdoctoral scholar Li Tang also contributed to the study. The team members were two undergraduate students; Michael Geitner, an undergraduate researcher and PPG fellow, and Fabiola Agramonte, an undergraduate student from the University of Puerto Rico who contributed as part of the department’s Research Experience for Undergraduates (REU) program backed by the National Science Foundation (NSF).
The researchers found that introducing “good bacteria” in the RO system can suppress the growth of harmful bacteria, eventually controlling the formation of biofouling causing biofilms.
What we created is the first cell that is able to talk to itself to control biofilm formation. Through genetic engineering, we have developed a cell that is able to recognize when it has reached a certain density, and in turn, sends a response to stop making biofilms. We call it a ‘living reverse osmosis membrane’ or ‘LROM.’ The cell’s ability to limit its own thickness assures that the good biofilm itself does not cause fouling.
Thomas Wood, Endowed Biotechnology Chair Professor of Chemical Engineering, Penn State
The rate of growth of the self-controlled biofilms is very less than uncontrolled biofilms. In addition, introducing genetically engineered elements into E. coli can activate the self-controlled biofilms. In trails, the generation of Pseudomonas and Sphingomonas strains was successfully suppressed by these engineered E. coli, which also provided protection to the membrane at the same time. Pseudomonas and Sphingomonas strains are the two most common bacteria in RO systems
The proposed method of applying engineered biofilms in membrane systems may also be advantageous in terms of sustainability. Existing techniques use propriety chemicals known as biocides to treat biofouling. These biocides could be harmful to the environment, ultimately incurring high associated costs. Conversely, LROM approach provides a greener and safer option.
The research team has evaluated the capability of its approach in both short- and long-term experiments under different flux conditions. Going forward, the researchers plan to conduct larger-scale testing and explore the potential of their system in the field of biomedical devices.
Eventually we plan to test more than just our two reference bacteria and expand the potential applications for this technology. Theoretically, this method could be applied to biomedical devices and implants to decrease the likelihood of failure due to biofilm infections.
Manish Kumar, Assistant Professor of Chemical Engineering, Penn State
The research is funded by an NSF grant presented in 2014, including an REU supplement in 2015. A provisional patent on the technology was filed by Penn State in November 2015. The university is now looking for industrial partners for commercialization of this concept.
The researchers have described their work in a paper titled, “Living Biofouling-Resistant Membranes: A Model for the Beneficial use of Engineered Biofilms,” in the Proceedings of the National Academy of Sciences of the United States of America.