May 6 2016
Using a cooling technique developed by researchers at Harvard University, JILA physicists have improved the ability of their robust laser “combing” technique to determine the structures of large, complicated molecules present in pharmaceuticals, fuels, explosives, and the gases surrounding stars.
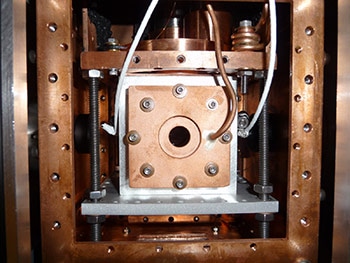 This JILA instrument uses a frequency comb to detect large, complex molecules, based on the precise frequencies, or colors, of light they absorb. The molecules are chilled and probed inside this chamber at temperatures near absolute zero. (Photo credit: Spaun/JILA)
This JILA instrument uses a frequency comb to detect large, complex molecules, based on the precise frequencies, or colors, of light they absorb. The molecules are chilled and probed inside this chamber at temperatures near absolute zero. (Photo credit: Spaun/JILA)
The JILA-Harvard work further extends the capability of spectroscopy, which studies light-matter interactions, a sought-after phenomenon in a number of fields, including physics, chemistry, imaging, remote sensing and astronomy.
The results of the study co-authored by the Harvard University researchers have been reported online in the Nature journal.
JILA is a collaboration between the National Institute of Standards and Technology (NIST) and the University of Colorado Boulder.
The tabletop-sized instrument developed by JILA is based on a laser frequency comb, which is a “ruler of light” honored in the 2005 Nobel Prize in Physics. The original molecule-detection apparatus was developed by JILA researchers a decade before. Since then, the researchers have used the system for a variety of applications, including detection of impurities in semiconductors, determination of trace gases in the atmosphere, and breath analysis to determine diseases.
The combing technique involves bouncing the comb laser light back and forth in a hollow chamber or cavity, where the molecules have been placed. The absorption of a small amount of the comb light at specific frequencies where the molecular rotation and vibration take place helps identify the unique “fingerprint” of each molecule in the absorption patterns along the thousands of comb frequencies. Although the technique has high sensitivity and specificity, the technique is limited to explore small and simple molecules composed of fewer than 10 atoms. This is due to the fact of existence of small molecules in millions of rotation and vibration states, with each having a different energy level. This, in turn, makes it difficult to identify their signals.
Being able to detect and unambiguously identify large molecules has been a longstanding goal. First, it provides fundamental insights into molecular structure and dynamics. Second, it allows us to understand increasingly complex systems. Third, for applications ranging from breath analysis to explosives detection, the capability to detect large molecules has been missing.
Jun Ye, JILA/NIST Fellow
The original JILA instrument probed molecules at room temperature, and Harvard’s helium buffer gas cooling technique is integrated into the upgraded system. The molecules are cooled down by the buffer gas to a temperature close to absolute zero (roughly -265°C or -445°F). As a result, their speed and rotation are considerably reduced, simplifying and strengthening the absorption signals. This largely improves the ability to determine the molecules. Using the buffer gas system, the molecules can be probed for over 10 ms, which is a period a thousand-fold longer compared to other cold molecule research systems. This capability enables the upgraded system to track cold chemical reactions.
Instead of just a glob of mountains in the signals, you can actually start to see the individual trees.
Jun Ye, JILA/NIST Fellow
The ability to probe several frequencies simultaneously provides the combing technique with an efficiency a thousand times greater than conventional single-frequency laser spectroscopy, where absorption signals are recorded one frequency at a time. The high precision of the comb also provides superior sensitivity and accuracy to the JILA technique compared to traditional broadband spectroscopy utilizing white-light sources.
Using the upgraded system, the JILA team identified organic compounds consisting of carbon-hydrogen bonds, which are capable of rotating and vibrating in multiple ways, including twisting, wagging, rocking, scissoring, and stretching. The enhanced system allowed the team to detect the absorption patterns of multiple vibrations of carbon-hydrogen bonds with superior resolution in four complex molecules rapidly, within 30 minutes to several hours:
- nitromethane (7 atoms, a model system for analysis of complex internal vibrations), utilized in making fuels, explosives, and pharmaceuticals;
- naphthalene (18 atoms), utilized in mothballs and found in interstellar space;
- hexamethylenetetramine (22 atoms), a sought-after molecule in astronomy and is utilized to make pharmaceuticals, plastics and explosives RDX; and
- adamantane (26 atoms), whose derivatives are used in lubricants and drugs
According to Ye, the JILA instrument could be helpful for scientists to analyze and gain knowledge about huge structures like buckyballs, which consist of molecules of 60 carbon atoms. In addition to probing new molecular species, chemical reactions can be studied in real time using this technique, allowing the monitoring of the behavior of highly reactive “free radicals” that can be found in human breath and explosives.
The Defense Advanced Research Projects Agency, Air Force Office of Scientific Research, NIST, and the National Science Foundation funded the research work.