Sep 14 2016
 CREDIT: Universität Basel
CREDIT: Universität Basel
On-surface chemical reactions have the potential to lead to the development of novel chemical compounds that are yet to be synthesized by solution chemistry. A high-resolution atomic force microscope can be used to study the first-step, second-step, and third-step products in detail.
This has been illustrated in Nature Communications by scientists from the Swiss Nanoscience Institute and the Department of Physics at Basel University and their colleagues from Finland and Japan.
In a number of nanotechnology applications, separate molecules are positioned on surfaces to fulfill particular functions, such as emitting a light signal or conducting an electrical current. Scientists will ideally synthesize these sometimes extremely complicated chemical compounds directly on the surface.
Ultra-high-resolution atomic force microscopes will help to provide step by step assistance for the on-surface chemical reactions. The collected data helps them to estimate the exact molecular structure and the energetics along the path.
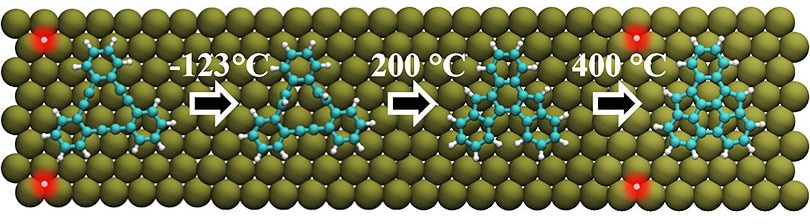
Colleagues of Professor Ernst Meyer from the University of Basel selected a molecule comprising of three benzene rings attached by a triple bond for their experiments. The molecules position themselves in a continuous pattern when this particular molecule is applied to a silver surface, however, a chemical reaction does not occur.
Copper as a Catalyst
The molecules are found to react at a temperature of -123°C on a copper surface. The precursor molecule, catalyzed by the copper atoms, integrates two hydrogen atoms in order to adjust its spatial arrangement and structure. Heating the sample to 200°C, results in a reaction step where two pentagonal rings are developed. Increasing the temperature to 400°C cuts the hydrogen atoms and creates a further carbon-carbon bond.
The final two reaction steps lead to the development of aromatic hydrocarbon compounds, which had earlier not been synthesized in solution chemistry.
These experiments were performed in ultra-high vacuum conditions and also monitored the synthesis using a high-resolution atomic force microscope with a carbon monoxide terminated tip. The exact molecular structure, corresponding to the microscope images, was generated by comparative computer calculations.
Tailored Nanostructures
The global research team used these experiments to highlight that on-surface chemistry can help to develop novel products.
This extremely pure form of chemistry provides us with tailored on-surface nanostructures that can be used in a variety of ways.
Ernst Meyer, Professor, University of Basel
In the discussed example, the copper surface works as a catalyst; the chemical reaction of the precursor molecules is monitored by adding heat and can also be monitored through atomic force microscopy.