Jan 16 2017
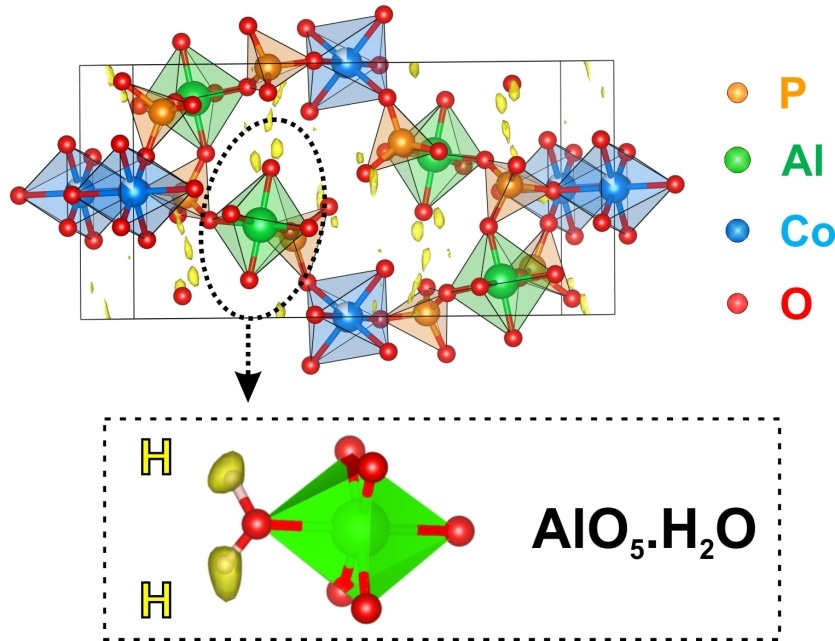 Representation of the structure of a cobalt aluminophosphate, superimposed onto a map showing maxima (in yellow) associated with the hydrogen positions, after analysis of the electron diffraction data. Credit: P. Boullay�CRISMAT (CNRS/Ensicaen/Unicaen).
Representation of the structure of a cobalt aluminophosphate, superimposed onto a map showing maxima (in yellow) associated with the hydrogen positions, after analysis of the electron diffraction data. Credit: P. Boullay�CRISMAT (CNRS/Ensicaen/Unicaen).
Although diffraction-based analytical techniques are widely employed in laboratories, they are still not very successful in analyzing samples that are smaller than a micrometer.
Scientists from the Laboratoire catalyse et spectrochimie (CNRS/Ensicaen/Unicaen), the Laboratoire de cristallographie et sciences des matériaux (CNRS/Ensicaen/Unicaen), and the Academy of Sciences of the Czech Republic have triumphantly employed electron diffraction to uncover the nanocrystal structure.
The high sensitivity of their technique has even enabled the position of hydrogen atoms to be located for the first time. Locating the position of hydrogen atoms is highly important to access the size of cavities in porous materials or morphology of the molecules. This research was published on 13 January 2017, and has been reported in the front page of the journal Science.
The prominent technique used for acquiring the atomic structure of crystalline solids crucial for discerning the properties of reactional mechanisms, materials, or biomolecules such as DNA or proteins is diffraction of neutrons or X-rays by crystals. Yet, this technique mandates the use of crystals of the order of a millimeter for neutrons, and a micrometer for X-rays.
Nanosized samples can be analyzed using electron diffraction, resulting from the strong interaction of these charged particles with the material. However, one of the major drawbacks of this method is that the occurrence of numerous diffractions minimizes the quality of the acquired results.
The kinematical theory of diffraction proposes that the diffracted particles are subjected to a single diffraction event. Although this estimation greatly simplifies analysis for neutrons and X-rays, it does not hold good for electrons. Therefore, it is necessary to employ the dynamical theory of diffraction, which considers the fact that electrons can be diffracted for numerous times. This requires a particular form of processing as well as a complex and lengthy analysis.
A new application of the dynamical theory for analyzing electron diffraction data has made the determination of the structures of an inorganic compound, a cobalt aluminophosphate, and an organic compound, paracetamol, possible. The exceptional sensitivity of this technique enables the position of the lightest atoms such as hydrogen atoms to be uncovered.
It is of crucial to reveal their position for accessing weak interactions in the material, the morphology of organic molecules, and the size of cavities in porous inorganic materials. It has been shown that the structure of the various compounds forming very small crystals can now be resolved up to its finest detail by localizing hydrogen atoms.
This research has opened a new path for large-scale usage of electron diffraction to ascertain the structure of crystals that cannot be determined by neutron or X-ray diffraction.