Jan 31 2017
 Physics Today. � 2017 EPFL
Physics Today. � 2017 EPFL
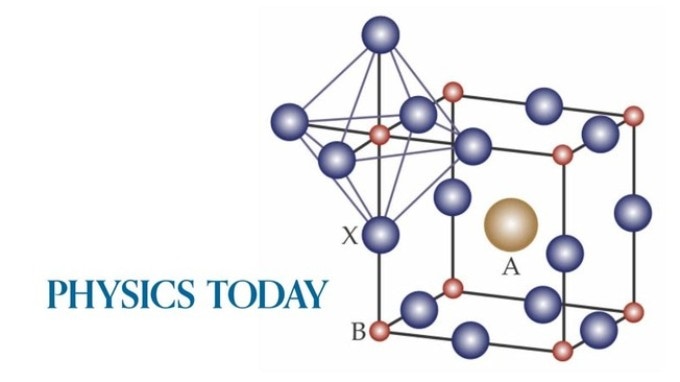
Recently, certain members of the class of crystalline materials called perovskites have shown immense promise for optoelectronic applications.
The chemical formula for perovskites is ABX3, where A and B are cations and X is an anion. Crystals that combine an organic cation, lead as the second cation, and a halogen anion make for solar cells of extremely high efficiency despite rather modest charge-carrier mobilities. However, the fate and nature of the photoexcited charge carriers continue to have little understanding.
A Swiss team, headed by Majed Chergui of the Swiss Federal Institute of Technology in Lausanne, recently peeled back some of that mystery. The team analyzed two inorganic perovskites, CsPbBr3 and CsPb(ClxBr1–x)3, at the Swiss Light Source using time-resolved x-ray absorption spectroscopy.
The researchers studied each element independently with a resolution of 80 ps by tuning the energy of the pulsed x rays. The researchers discovered that for both materials the excited electrons are delocalized in the conduction band, but the holes left behind in the valence band are localized at the Br sites.
The Br signal comprised of one component that quickly decayed and one that decayed slowly. A similar biexponential decay is shown by the photoluminescence kinetics of organic–inorganic perovskite solar cells. In the meantime, the Cs+ cations revealed no evidence of being involved in the charge transport, which highlights that organic cations would likewise be uninvolved.