Mar 21 2017
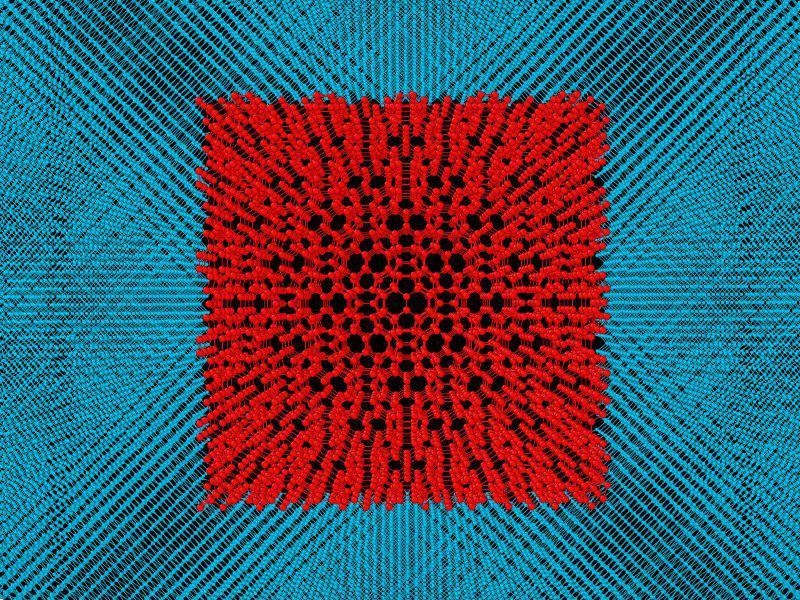 Model of fullerite inside diamond. Figure provided by A. Kvashnin
Model of fullerite inside diamond. Figure provided by A. Kvashnin
The structure of a new material based on fullerite and a single crystal diamond has been simulated by physicists in order to demonstrate how this material can acquire ultrahigh hardness. This discovery allows the estimation of the potential conditions for acquiring ultrahard materials. The results were featured in the Carbon journal.
Generally, fullerite is a molecular crystal made up of fullerene molecules at its lattice nodes. Fullerenes are a form of molecular carbon where carbon atoms form a sphere. It was first synthesized over 30 thirty years ago, and the Nobel Prize was awarded for this discovery.
It is possible to pack carbon spheres in fullerite a number of ways, and the material’s hardness strongly depends on how the fullerenes are bound to each other.
Reasons as to why fullerite becomes an ultra-hard material were explained by a team of scientists from the Moscow Institute of Physics and Technology (MIPT), the Skolkovo Institute of Science and Technology (Skoltech), the National University of Science and Technology (MISIS) and the Federal State Budgetary Scientific Institution Technological Institute for Superhard and Novel Carbon Materials (FSBSI TISNCM, Moscow, Troitsk) headed by Prof. Leonid Chernozatonskii from the Institute of Biochemical Physics (IBCP) of the Russian Academy of Sciences.
When we started to discuss this idea, I was working at TISNCM. There, in 1998, a group of scientists headed by Vladimir D. Blank obtained a new material based on fullerenes — ultrahard fullerite, or “tisnumit”. According to the measurements, this new material could scratch diamond, that is, it was in fact harder than diamond.
Alexander Kvashnin, Candidate of Physics and Mathematics, MIPT
The substance obtained was not a single crystal material; it contained amorphous carbon and 3D-polymerized molecules of С60. However, its crystal structure is yet to be completely determined. The fullerene molecule is made up of exceptional mechanical rigidity.
Under normal conditions, the fullerite crystal is relatively a soft material, but becomes harder than diamond when under pressure because of the 3D polymerization. The reason why this material becomes ultrahard is yet to be discovered, even though it has been synthesized and studied for over 20 years. Several models have been developed to demonstrate the polymerization of fullerenes into fullerite.
Prof. Leonid A. Chernozatonskii proposed one of the models. The model’s X-ray diffraction pattern rightly agrees with experimental data and should possess high volumetric bulk modulus, which is several times higher than the diamond value. However, such interesting properties are not displayed by the relaxed structure of the model.
We based our analysis on that model and the experimentally known fact that if you apply high pressure, more than 10 GPa to fullerene powder, and heat it above 1800 К, you obtain a polycrystalline diamond. The idea was to combine these two facts. On the one hand, a super-hard fullerite material, and on the other hand, under pressure, fullerenes turn into a polycrystalline diamond.
Alexander Kvashnin, Candidate of Physics and Mathematics, MIPT
According to the scientists, part of the fullerite turned into diamond when placed under pressure, while the other part continued to be as fullerite, but in a state compressed within the diamond. The model was simplified by taking the fullerite crystal structure proposed by Prof. Chernozatonskii and placing it inside a single crystal diamond.
This was following by studying this composite material. The idea here emphasized on the need to compress the fullerite inside diamond. It is an established fact that the mechanical and elastic properties of the material would increase in the compressed state. Diamond would act as a shell, keeping the compressed fullerite inside in order to preserve all those properties.
Small models comprising of 2.5 nm fullerite grain inside the diamond shell, which is 1 nm thick, were first analyzed in the study by the scientists. This type of a small model failed to comply with the experimental data. The composites were then modeled by the researchers, resulting in an increase in the size of fullerite up to 15.8 nm, and the diamond shell’s thickness remained the same.
The changes in the X-ray diffraction spectrum illustrated that the spectrum was brought closer to the experimental data by the increase in the size of the fullerite.
Following a comparison of the spectra, it was assumed that most likely in the experiment, they had attained an amorphous carbon medium with a hydrostatically compressed fullerite inside, while the model dealt with a diamond comprising of a fullerite. The calculated spectrum highlighted that the new model correlated extremely well with the experimental data.
The developed model will help us to understand the nature of its unique properties and to help to systematically synthesize the new ultra-hard carbon materials, as well as to contribute to the further development of this promising field of science.
Pavel Sorokin, Doctor of Physical and Mathematical Science, TISNCM, MISIS, MIPT
Fullerite by itself is not very hard. Compared to diamond, the bulk modulus of fullerite is 1.5 times less than that of diamond. However, the bulk modulus of fullerite dramatically increases when fullerite is compressed. It is essential that the fullerite should always remain in such a compressed state in order to preserve this improved bulk modulus. The scientists can make use of the results of simulations in order to perform targeted experiments to attain an ultra-hard material.