Oct 20 2017
Rechargeable batteries developed from magnesium, in place of lithium, possess the ability to increase the driving range of the electric vehicle by loading more energy into small-sized batteries. However, unexpected chemical obstacles have decelerated the scientific advancement.
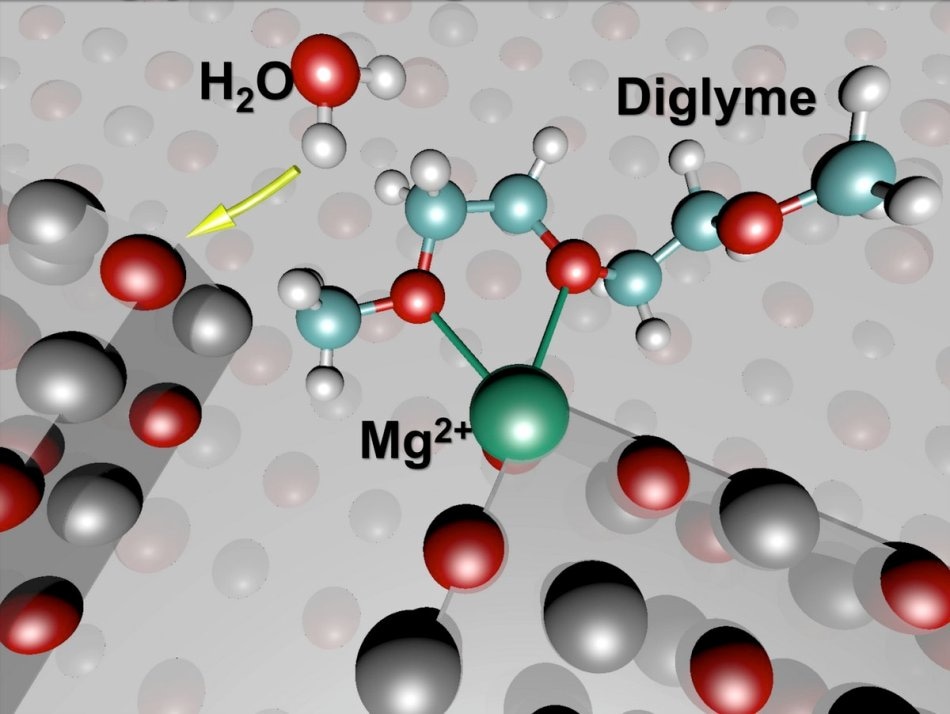 These molecular models show the initial state of battery chemistry that leads to instability in a test cell featuring a magnesium (Mg) anode. CREDIT: Berkeley Lab.
These molecular models show the initial state of battery chemistry that leads to instability in a test cell featuring a magnesium (Mg) anode. CREDIT: Berkeley Lab.
The areas where solid comes into contact with liquid, places in which oppositely charged battery electrodes react with the enclosing chemical mixture called electrolyte, are the challenging spots.
Scientists from the U.S. Department of Energy’s Joint Center for Energy Storage Research, headed by researchers at Lawrence Berkeley National Laboratory (Berkeley Lab), have found out an surprising set of chemical reactions that involve magnesium. These reactions cause the degredation in the performance of the battery even before the battery is charged.
The findings could be relevant to other battery materials, and could steer the design of next-generation batteries toward workarounds that avoid these newly identified pitfalls.
The researchers adopted theoretical modeling, X-ray experiments, and supercomputer simulations to have an in-depth knowledge of the chemical composition of a liquid electrolyte located just tens of nanometers from an electrode surface that affects the performance of the battery. The outcomes of the study have been reported in the online publication of the Chemistry of Materials journal.
The battery being test contained magnesium metal, used as the negative electrode, or anode, which was in contact with a liquid electrolyte called diglyme, and a dissolved salt, that is, Mg(TFSI)2.
The combination of materials were thought to be well-suited and nonreactive during the resting state of the battery. However, experiments performed by using a synchrotron (an X-ray source) at Berkeley Lab’s Advanced Light Source (ALS) found that this wasn't the case and took the research in a different direction.
People had thought the problems with these materials occurred during the battery’s charging, but instead the experiments indicated that there was already some activity.
David Prendergast, Director, The Theory of Nanostructured Materials Facility, The Molecular Foundry and one of the lead authors of study
“At that point it got very interesting,” stated Prendergast. “What could possibly cause these reactions between substances that are supposed to be stable under these conditions?”
Scientists at the Molecular Foundry created elaborate simulations of the place at which the electrode and electrolyte come into contact (i.e. the interface), suggesting that spontaneous chemical reactions must not take place even under suitable conditions. However, the simulations did not explain all the chemical details.
“Prior to our investigations,” stated Ethan Crumlin, an ALS scientist who was the co-lead author of the study with Prendergast and who coordinated the X-ray experiments, “there were suspicions about the behavior of these materials and possible connections to poor battery performance, but they hadn’t been confirmed in a working battery.”
In the case of commercially popular lithium-ion batteries which power various portable electronic devices (e.g. laptops, mobile phones, and power tools) and a broad range of electric vehicles. Lithium ions (or lithium atoms that get charged after losing an electron) are made to shuttle to and fro between the two electrodes of the battery. Such electrode materials are porous at the atomic level and are alternatively loaded with or emptied of lithium ions when the battery gets charged or discharged, respectively.
The negative electrode in such batteries is usually formed of carbon, with restricted potential to store the lithium ions when compared to other materials.
Therefore, when the density of stored lithium is increased by adopting a different material, the resulting batteries would be smaller, light-weight, and more powerful. For instance, if lithium metal is used in the electrode, more lithium ions are loaded in the same space. However, it is highly reactive and gets ignited during exposure to air, and mandates further studies to learn the best way to load and protect it to ensure long-term stability.
When compared to lithium metal, magnesium metal has higher energy density, indicating that it could theoretically store more energy in a same-sized battery by using magnesium in the place of lithium.
In comparison with lithium, magnesium is highly stable. When magnesium reacts with oxygen and moisture in air, a self-protecting “oxidized” layer is formed by its surface. However, inside a battery, the oxidized layer is considered to degrade the efficiency and cut short the working life of the battery. Therefore, the team is searching for methods for preventing the formation of the oxide layer.
In order to perform in-depth investigation of the formation of this layer, the researchers adopted a distinctive X-ray method called ambient pressure X-ray photoelectron spectroscopy, or APXPS, recently formulated at the ALS. This innovative method is sensitive to the reactions taking place at the solid-liquid interface, rendering it to be an optimal tool for investigating battery chemistry at the electrode’s surface, the point at which it is in contact with the liquid electrolyte.
X-ray results gained before passing a current into the test battery indicated signs of chemical degradation of the electrolyte, particularly near the interface of the magnesium electrode. The discovery compelled the team to reconsider their molecular-level image of these materials and the way they react.
They deduced that the thin, self-stabilizing oxide surface layer formed on the magnesium has flaws and contaminants that lead to undesirable reactions.
It’s not the metal itself, or its oxides, that are a problem, it’s the fact you can have imperfections in the oxidized surface. These little disparities become sites for reactions. It feeds itself in this way.
David Prendergast, Director, The Theory of Nanostructured Materials Facility, The Molecular Foundry and one of the lead authors of study
Another round of simulations, predicted that there could be probable flaws in the oxidized magnesium surface, it demonstrated that flaws in the oxidized surface layer of the anode could lead to exposure of the magnesium ions which then function as traps for the molecules of the electrolyte.
When free-floating hydroxide ions, or molecules including a single oxygen atom connected to a hydrogen atom formed when trace amounts of water react with the magnesium metal, come into contact with these surface-bound molecules, they react with the molecules.
This leads to wastage of the electrolyte and thus battery dry-out in the long run. Moreover, the reaction products degrade the surface of the anode, resulting in a further decline in the functioning of the battery.
The experimental as well as theoretical researchers in the team performed a number of iterations backwards and forwards to create a model that correlated with the X-ray measurements. The attempts were compounded by millions of hours’ worth of computing power at the Lab’s National Energy Research Scientific Computing Center.
The team indicated the significance of the access to nanoscale expertise, X-ray methods, and computing resources at the same Lab.
The outcomes of the study can prove to be applicable even for other kinds of battery materials, such as prototypes based on aluminum or lithium metal. Prendergast stated that “This could be a more general phenomenon defining electrolyte stability.”
Crumlin further stated, “We’ve already started running new simulations that could show us how to modify the electrolyte to reduce the instability of these reactions.” Similarly, he also noted that the surface of the magnesium can be prospectively customized to decrease or prevent specific undesirable chemical reactivity.
Rather than allowing it to create its own interface, you could construct it yourself to control and stabilize the interface chemistry, right now it leads to uncontrollable events.
Ethan Crumlin, an ALS scientist who was the co-lead author of the study
Berkeley Lab’s Advanced Light Source, Molecular Foundry, and National Energy Research Scientific Computing Center are DOE Office of Science User Facilities accessible to visiting scientists at the national as well as global levels.
The team included scientists from the Joint Center for Energy Storage Research at Berkeley Lab and Sandia National Laboratories in New Mexico comprised, and also researchers from the University of Maryland and from the Shanghai Institute of Microsystem and Information Technology in China. The U.S. Department of Energy’s Office of Basic Energy Sciences supported the study.