Dec 6 2017
Carbon footprint can be drastically reduced by replacing the everyday gas guzzler with a hydrogen fueled car. So why cannot everyone make the switch?
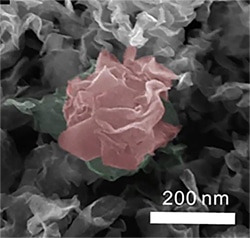 These inorganic “flowers,” color added, were created by Sandia National Laboratories researcher Stanley Chou and University of California, Merced colleague Vincent Tung in a spray-printing process that uses molybdenum disulfide to create a “flowering” hydrogen catalyst far cheaper than platinum and reasonably close in efficiency. (Image courtesy of Sandia National Laboratories)
These inorganic “flowers,” color added, were created by Sandia National Laboratories researcher Stanley Chou and University of California, Merced colleague Vincent Tung in a spray-printing process that uses molybdenum disulfide to create a “flowering” hydrogen catalyst far cheaper than platinum and reasonably close in efficiency. (Image courtesy of Sandia National Laboratories)
One of the reasons why individuals do not make the switch is because of the expensive platinum catalyst needed for operating hydrogen fuel cells in an efficient manner.
Research headed by Sandia National Laboratories and the University of California, Merced focused on bringing down the cost of hydrogen fuel cells by using a majorly cost-effective compound in order to develop an uneven surface that looks like the leaves of a plant. The additional area enables catalyzing hydrogen almost as efficiently as platinum.
Lead researchers Stanley Chou, a Sandia materials scientist, and UC Merced’s Vincent Tung have employed a joint patent for the spray-printing process, which makes use of inexpensive molybdenum disulfide. The increased surface area of the rippling “leaf” develops three times as many catalytic contact points as various other molybdenum disulfide structures, and the new creation is capable of handling higher temperatures than platinum without gumming up and sintering the cell.
The work is part of an effort to power hydrogen-fueled cars in a cheaper and desirable manner as they emit water rather than carbon dioxide or carbon monoxide.
Nature as an ally
The production method makes use of nature as an ally instead of considering it to be a hindrance, Chou said. “In traditional thinking, forces such as gravity, viscosity and surface tension must be overcome to achieve the manufactured shapes you desire. We thought, instead of thinking these forces as limitations, why not use them to do something useful? So, we did.”
Tung explained that the method employs natural processes to develop materials for majorly inexpensive fuel cell terminals to release hydrogen. “The printing process also allows for continued deposition, with the ability to scale for industry,” he said.
The team mixed molybdenum disulfide with water and employed the printing process in order to expel micron-size droplets into an enclosed area about 2 feet high. As they dropped, the droplets initially separated into nanoscopic subunits. These dried further as they fell, their shrinking volume generating an uneven 3D surface similar to the leaves of plants, with small ridges, canals, caves, hills and tunnels. The leaves, landing on a substrate and on each other, were still adequately moist such that they could bond as though fixed at critical points by small droplets of glue. The nanostructures thus did not lose their individuality but instead maintained their identities and thus produced small tunnels between and within them that permitted unexpected access for atoms of hydrogen to pursue their freedom from chemical bonds.
The inspiration for developing a bio-inspired 3D form developed from studying the cuticle folding process, a mechanism employed by plants for controlling permeability and diffusion on leaf surfaces, Chou said.
“We see our catalyst as an inorganic material acting like a plant. The nanostructures, like leaves, are varied in shape, with tiny rises and falls,” he said. “The structures take in an external material to produce hydrogen rather than oxygen, and one day may be powered by sunlight.” Extremely low-voltage electricity does the job at the moment.
Doubts regarding the strength of structure produced in such a serendipitous manner, Tung recounted, were solved when a 170-pound student unintentionally trod upon one of the first molybdenum disulfide-catalyst creations when it fell to the floor accidentally. A few hundred nanometers thick, it then rested upon a centimeter-square carbon substrate but was otherwise left unprotected. Electromicroscopic investigation proved the small structure to be undamaged. The “leaves” have also proved to be long lasting, continuing to generate hydrogen for six months.
The work is considered to be the subject of a technical article featured online in the journal Advanced Materials. Researchers from King Abdullah University of Science and Technology, Lawrence Berkeley National Laboratory and Yale University have also contributed to the article.
The Department of Energy’s Office of Science funded the work at Sandia. A university startup fund supported the work at UC Merced.