Jun 5 2018
Single-atom catalysis is a developing process of catalysis. Yet, there still exist various basic problems that have to be overcome.
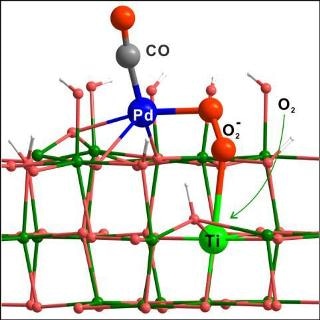 Image Credit: Science China Press
Image Credit: Science China Press
A recent study has shown that, apart from acting as ligands that stabilize single-metal atoms, metal atoms that are close to catalytic metal atoms and can directly take part in the activation of reactants, thereby resulting in a notable vicinal impact for promoting catalysis of an atomically dispersed metal catalyst.
The outcomes of the study have been reported in the paper titled “A vicinal effect for promoting catalysis of Pd1/TiO2: supports of atomically dispersed catalysts play more roles than simply serving as ligands” (cover article in Science Bulletin, 2018, 11) by Professor Nanfeng Zheng’s team from Xiamen University.
Based on an earlier study, the researchers analyzed the catalytic performances of two atomically dispersed catalysts (supported on TiO2 of distinctive surface properties) that have unique local coordination structures.
The two catalysts were found to exhibit considerably distinctive catalytic performances, despite the fact that their electronic characteristics were very similar. Systematic experimental and theoretical calculations performed by the team showed that the atomic Pd-O-Ti(III) interface on the TiO2-supported Pd single-atom catalyst readily activated O2 into superoxide at ambient temperature to initiate the catalytic oxidation of CO at low temperatures.
Such a distinctive oxygen activation pathway also rendered the catalyst very active in the oxidative combustion of greenhouse gas (methane) and harmful volatile organic compound (toluene).
The most difficult part of the research was the identification of the atomic structure of Pd-O-Ti(III), which was albeit finely dealt with by the extensive cooperation among Xiamen University, Institute of Physics at Chinese Academy of Sciences, and the Shanghai Synchrotron Radiation Facility and Dalhousie University, which enabled the catalytic mechanism at the molecular level to be fully understood.
The research also showed that the active center of an atomically dispersed metal catalyst should not be restricted to only the dispersed metal atoms.
Probabilities are more for the metal atoms of the support to be located close to the catalytic metal centers could be directly involved in the catalysis, thereby having a vital role in promoting catalysis.
Put differently, the support’s metal atoms could be part of the catalytically active centers. Hence, while designing efficacious atomically dispersed metal catalysts, the impact and thus significance of metal precursors, supports, and preparation should never be disregarded.
The outcomes of this study help well in explaining the reason for catalysts with the same metal atomically dispersed on distinctive supports to sometimes exhibit considerably distinctive performances in the same reactions.
Meanwhile, certain phenomena, such as the significance of pre-catalysis reduction process as well as the promoting effect of alkali metal ions, detected in the catalysis community can also be perceived from the point of view of the vicinal impact.
This research should offer theoretical guidance for rationally designing and synthesizing efficient atomically dispersed metal catalysts, and also initiate more research works for gaining insights into catalytic mechanisms at the molecular level.