Aug 13 2018
Surface atoms are not all identical when a metal surface catalyzes a chemical process. EPFL researchers have closed the gap between lab and plant by examining methane dissociation on the (211) crystal face of platinum.
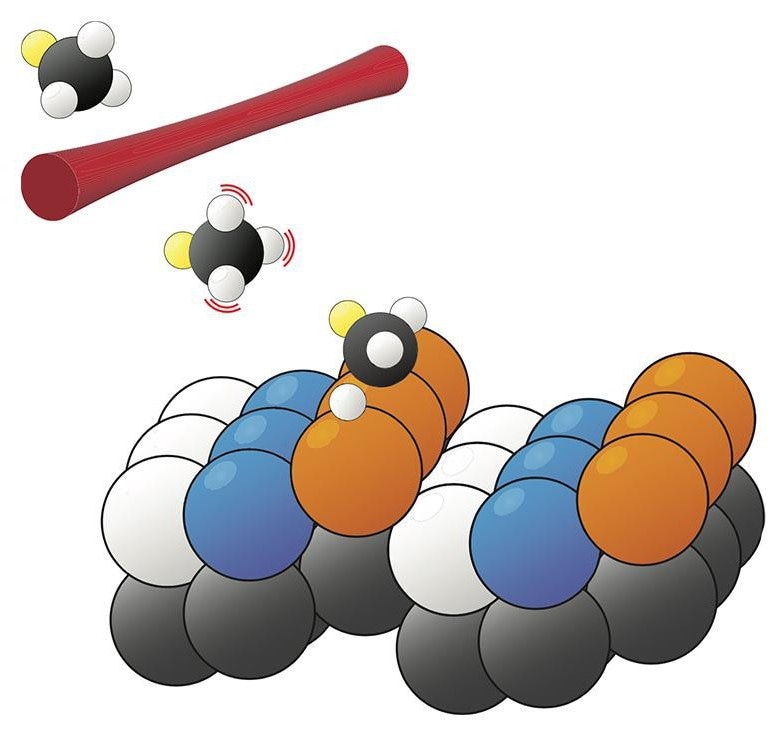 The bond-selective and surface-site-specific dissociation of deuterated methane on a Pt(211) surface (Image credit: R. Beck/EPFL)
The bond-selective and surface-site-specific dissociation of deuterated methane on a Pt(211) surface (Image credit: R. Beck/EPFL)
Back in 1909, Fritz Haber made a discovery that would revolutionize the world: With the aid of a solid iron catalyst, he could break atmospheric nitrogen into its constituent atoms and unite them with hydrogen to form ammonia. The fertilizers made from that synthetic ammonia boosted agricultural productivity many times over and allowed Earth’s increasing population to be nourished.
Industrial surface catalysis - currently extended to many more reactions - is a muddled process. The catalytic surfaces are rough and uneven, and hot reactant molecules push one another and travel in all directions. Chemical physicists get to understand the reactions by streamlining them: The craggy catalysts and pressurized reaction vessels in a chemical plant are swapped with smooth crystalline surfaces and rarefied atmospheres in the lab. The experiments have produced some vital results, including the comprehensive mechanism of the Haber process, but they are very different from real-world conditions.
Rainer Beck, of the Swiss Federal Institute of Technology in Lausanne, and his colleagues are closing the gap between lab and plant by examining methane dissociation on the (211) crystal face of platinum. With its regularly spaced steps, Pt(211) has three different types of surface sites, illustrated in the figure in orange, blue, and white. Earlier, experiments could measure just the average reaction output of the entire surface. But at the beginning of this year, Beck and company demonstrated that the reaction product, CH3, had measurably varied vibrational resonances depending on the surface site where it was found. By observing the vibrational spectrum of the entire surface and resolving it into site-specific components, the scientists investigated the catalytic activity of each type of site. Qualitatively, the results matched with expectations: The step sites (orange) are more active than the terrace sites (blue), and the corner sites (white) do not influence the reaction at all.
Currently, Beck and colleagues have incorporated a new layer of complexity. By substituting CH4 with deuterated methane, CH3D, they have developed a system in which over one dissociation reaction is possible: The bond that breaks could be either C–H or C–D. The ability to direct such a reaction toward a preferred set of products is an age-old goal. With deuterated methane, one can develop a preference for C–H bond dissociation by preparing the reactant molecules in a state in which the C–H bonds vibrate powerfully but the C–D bond does not. But the degree of that preference displays a complex dependence - which Beck and colleagues have at present disentangled—on the reaction site as well as the reactant speed.
The Lausanne experimenters are involved with a number of theory groups to change their results into an improved predictive understanding of surface reactions. Also, they are spreading out their experiments to even more intricate surfaces, such as Pt(531), whose steps are zigzag in shape.