Nov 8 2018
A closer investigation of materials that make up conventional solar cells exposes a virtually rigid arrangement of atoms with minimal movement. But in hybrid perovskites, a favorable group of solar cell materials, the arrangements have better flexibility and atoms dance freely about, an effect that influences the performance of the solar cells but has been hard to measure.
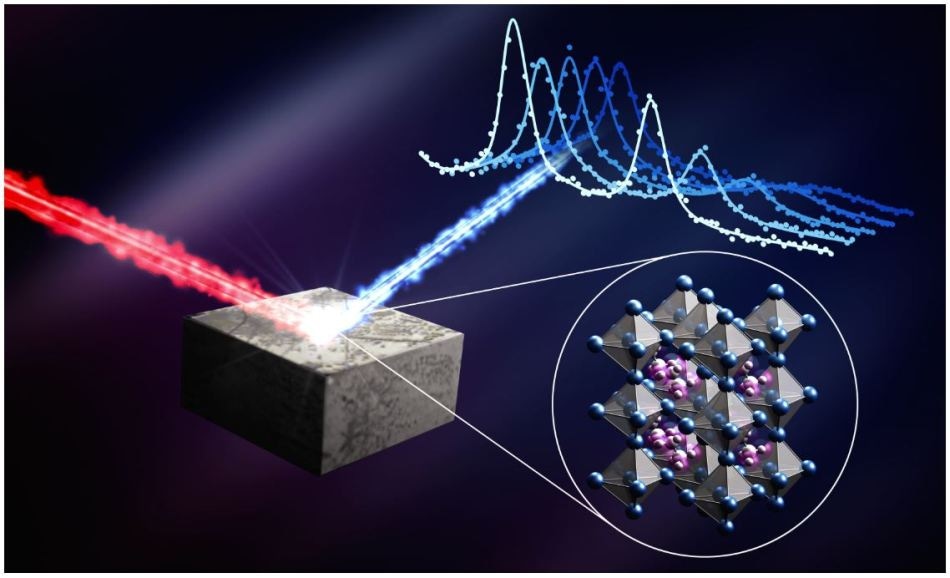 When the researchers scattered neutrons off the perovskite material (red beam) they were able to measure the energy the neutrons lost or gained (white and blue lines). Using this information, they were able to see the structure and motion of the atoms and molecules within the material (arrangement of blue and purple spheres). (Credit: Greg Stewart/SLAC National Accelerator Laboratory)
When the researchers scattered neutrons off the perovskite material (red beam) they were able to measure the energy the neutrons lost or gained (white and blue lines). Using this information, they were able to see the structure and motion of the atoms and molecules within the material (arrangement of blue and purple spheres). (Credit: Greg Stewart/SLAC National Accelerator Laboratory)
In a paper published in the Proceedings of the National Academy of Sciences, an international team of scientists led by the U.S. Department of Energy’s SLAC National Accelerator Laboratory has developed a new insight of those wild dances and how they impact the working of perovskite materials. The results could elucidate why perovskite solar cells are so efficient and aid the mission to design hot-carrier solar cells, a theorized technology that would virtually double the efficiency parameters of conventional solar cells by converting more sunlight into practical electrical energy.
Piece of the puzzle
Perovskite solar cells, which can be created at room temperature, offer a cheaper and possibly better performing substitute to conventional solar cell materials like silicon, which have to be produced at very high temperatures to exclude defects. However, a lack of insight regarding what makes perovskite materials so efficient at transforming sunlight into electricity has been a big obstacle to manufacturing even higher efficiency perovskite solar cells.
“It’s really only been over the last five or six years that people have developed this intense interest in solar perovskite materials,” says Mike Toney, a distinguished staff scientist at SLAC’s Stanford Synchrotron Radiation Light Source (SSRL) who led the research. “As a consequence, a lot of the foundational knowledge about what makes the materials work is missing. In this research, we provided an important piece of this puzzle by showing what sets them apart from more conventional solar cell materials. This provides us with scientific underpinnings that will allow us to start engineering these materials in a rational way.”
Keeping it hot
When sunlight reaches a solar cell, some of the energy can be used to push electrons in the material up to higher energy states. These higher-energy electrons are funneled out of the material, generating electricity.
But before this takes place, a major portion of the sun’s energy is lost to heat with some portion also lost during the extraction of usable energy or because of inefficient light collection. In a number of conventional solar cells, such as those designed with silicon, acoustic phonons—a sort of sound wave that spreads through material—are the main way that this heat is transported through the material. The energy is lost by the electron as heat restricts the efficiency of the solar cell.
In this research, theorists from the United Kingdom, led by Imperial College Professor Aron Walsh and electronic structure theorists Jonathan Skelton and Jarvist Frost, provided a theoretical outline for deducing the experimental results. They stated that acoustic phonons moving through perovskites would have short lifetimes because of the flexible arrangements of dancing atoms and molecules in the material.
Stanford chemists Hema Karunadasa and Ian Smith were successful in growing the large, specialized single crystals that were vital for this study. With the assistance of Peter Gehring, a physicist at the NIST Center for Neutron Research, the team scattered neutrons off these perovskite single crystals in a way that permitted them to retrace the motion of the atoms and molecules within the material. This lets them to exactly measure the lifetime of the acoustic phonons.
The researchers learned that in perovskites, acoustic phonons are extremely short-lived, lasting for only 10 to 20 trillionths of a second. Without these phonons transporting heat through the material, the electrons might remain hot and hold onto their energy as they are pulled out of the material. Harnessing this effect could potentially result in hot-carrier solar cells with efficiencies that are virtually twice as high as conventional solar cells.
Furthermore, this occurrence could explain how perovskite solar cells function so well regardless of the material being riddled with flaws that would capture electrons and diminish performance in other materials.
Since phonons in perovskites don’t travel very far, they end up heating the area surrounding the electrons, which might provide the boost the electrons need to escape the traps and continue on their merry way.
Mike Toney, Staff Scientist, SLAC’s Stanford Synchrotron Radiation Light Source (SSRL).
Transforming energy production
To further expand on this research, scientists at the Center for Hybrid Organic-Inorganic Semiconductors for Energy (CHOISE) Energy Frontier Research Center led by DOE’s National Renewable Energy Laboratory will examine this occurrence in more complicated perovskite materials that are known to be more efficient in energy devices. They hope to understand how altering the chemical make-up of the material impacts acoustic phonon lifetimes.
“We need to fundamentally transform our energy system as quickly as possible,” says Aryeh Gold-Parker, who co-led the study as a PhD student at Stanford University and SLAC. “As we move toward a low-carbon future, a very important piece is having cheap and efficient solar cells. The hope in perovskites is that they'll lead to commercial solar panels that are more efficient and cheaper than the ones on the market today.”
The study team also included researchers from NIST; the University of Bath and Kings College London, both in the UK; and Yonsei University in Korea.
SSRL is a DOE Office of Science user facility. This research was aided by the DOE’s Office of Science and the Solar Energy Technologies Office, the Engineering and Physical Sciences Research Council, the Royal Society, and the Leverhulme Trust.