Dec 13 2018
In association with Irish colleagues, I.M. Sechenov First Moscow State Medical University successfully developed a novel imaging technique for tissue engineering. The so-called “hybrid biosensor” scaffold materials, based on cellulose matrices labeled with calcium- and pH-sensitive fluorescent proteins, were created by the research team.
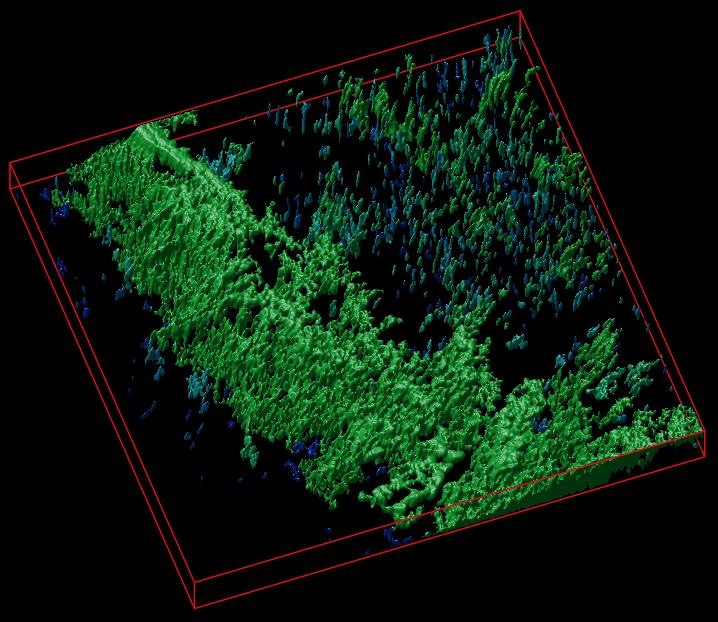 A 3D reconstruction of a cellulose matrix stained with a pH-sensitive biosensor. (Image credit: Dr R. Dmitriev)
A 3D reconstruction of a cellulose matrix stained with a pH-sensitive biosensor. (Image credit: Dr R. Dmitriev)
These types of materials make it possible to view the metabolism and other major biomarkers in the engineered artificial tissues through microscopy. The study results have been reported in the journal Acta Biomaterialia.
The success of tissue engineering builds on the application of scaffold matrices—materials that are capable of supporting the viability and directing the growth of tissues, cells, and organoids.
In basic and applied biomedical research, regenerative medicine, and tissue engineering, scaffolds are highly crucial and hold potential for developing novel therapeutics. Conversely, the ability “to see” what exactly occurs inside the scaffolds at the time of tissue growth presents a major research challenge.
“We developed a new approach allowing visualization of scaffold-grown tissue and cells by using labeling with biosensor fluorescent proteins. Due to the high specificity of labeling and the use of fluorescence microscopy FLIM, we can quantify changes in pH and calcium in the vicinity of cells,” stated Dr Ruslan Dmitriev, Group Leader at the University College Cork and Institute for Regenerative Medicine (I.M. Sechenov First Moscow State Medical University).
In order to attain the particular labeling of cellulose matrices, investigators applied popular cellulose-binding proteins. The application of extracellular calcium- and pH-sensitive biosensors makes it possible to study cell metabolism: Obviously, the extracellular acidification is directly linked to the balance of the glycolytic flux (release of lactate) and cell energy production pathways. In addition, it is a recurrent trademark of cancer as well as transformed cell types. Calcium, on the other hand, has a key role to play in the intracellular and extracellular signaling that impacts the growth and differentiation of cells.
The method was tested on various types of cellulose matrices (bacterial and created from decellularized plant tissues) through stem-cell-derived mouse small intestinal organoids and 3D culture of human colon cancer cells. The scaffolds informed on extracellular acidification changes and were utilized together with the study of real-time oxygenation of intestinal organoids. The data, thus obtained, can be presented in the form of color maps, matching to the areas of cell growth inside different microenvironments.
Our results open new prospects in the imaging of tissue-engineered constructs for regenerative medicine. They enable deeper understanding of tissue metabolism in 3D and are also highly promising for commercialisation.
Dr Ruslan Dmitriev, University College Cork; Institute for Regenerative Medicine, I.M. Sechenov First Moscow State Medical University
The study is a result of joint efforts between the University College Cork and Federal Science and Research Center “Crystallography and Photonics,” Russian Academy of Sciences.