Mar 25 2019
Scientists from Tokyo Metropolitan University have demonstrated that the tunable hydrophobic characteristic of dense siloxane gels is significantly linked to their catalytic activity, clearly showing how molecules with diverse hydrophobic nature at the molecular level interact differently with surfaces of differing hydrophobicity.
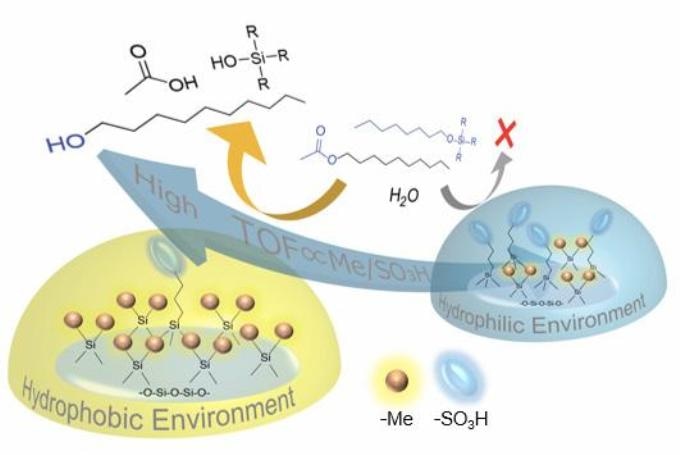 Simplified schematic showing how alkyl acetates with long tails cannot approach acidic sulfo sites (blue discs) in hydrophilic environments; however, they can approach hydrophobic environments with more methyl groups (small orange spheres), leading to a higher turnover frequency (TOF), a measure of how effective a catalyst is. (Image caption: Hiroki Miura)
Simplified schematic showing how alkyl acetates with long tails cannot approach acidic sulfo sites (blue discs) in hydrophilic environments; however, they can approach hydrophobic environments with more methyl groups (small orange spheres), leading to a higher turnover frequency (TOF), a measure of how effective a catalyst is. (Image caption: Hiroki Miura)
In addition, this is the first time a siloxane gel has been established to be highly effective for the reaction of silyl ethers, widely used as a protecting agent.
The word hydrophobic is derived from ancient Greek, “hydro” which means water and “phobia” which means fearing (opposite of hydrophilic). Therefore, a hydrophobic material is one which repels water; coatings for non-stick frying pans and smartphones are the household examples for such materials. Furthermore, hydrophobicity plays an important role in nature, for example, how certain animals and plants collect water from the atmosphere, and how long strands of DNA are packed well into chromosomes.
Recently, it has also been shown to be part of the function of acid catalysts, acidic materials which can accelerate chemical reactions, extensively employed in the petrochemical industry. While it was extensively known that more hydrophobicity results in better catalysis, it was not obvious why this was the case, because of the heterogeneous porous structure of the most common catalysts.
Therefore, a team of scientists headed by Dr Hiroki Miura and Prof Tetsuya Shishido from Tokyo Metropolitan University investigated the catalytic activity of a dense siloxane gel, a type of silicone rubber, with acidic sulfo groups linked. Notably, these gels can be covered with controlled amounts of acid groups as well as hydrophobic methyl groups, allowing precise control of hydrophobicity.
Furthermore, these gels are not porous, exhibiting a surface that is covered in only two main groups, enabling easier but more exact quantitation of the surface environment. The team investigated catalysis of the hydrolysis (bond breakage with water) of alkyl acetates, generally used for making perfumes, paints, and even plastics; they discovered that acetates with longer, more hydrophobic tails in their molecular structure took advantage of increased catalysis with a lower sulfo-to-methyl ratio.
In contrast, less hydrophobic molecules were catalyzed less effectively because of less available sulfo groups. They evidently show how the affinity of water to catalysis sites can obstruct the approach of different molecules; this may be exploited to yield selectivity as well as increased activity.
Moreover, the siloxane catalyst was used for the deprotection of silyl ethers, which are protecting groups, linked to groups which require protection from unnecessary reactions. They must be readily deprotected to make them accessible again. The team demonstrated, for the first time, that siloxane gel catalysts are highly effective in deprotecting silyl ethers, an important reaction step in general reactions like the building of artificial nucleotides (or DNA). By gaining more insights into how molecular environment is tied to function, the researchers expect that further chemical improvements to these catalysts may pave the road to new functions and applications.