Mar 26 2019
The reversible switching of macrocyclic molecules between a liquid and a solid phase upon exposure to vapor has been reported in the Journal of the American Chemical Society by researchers at Kanazawa University.
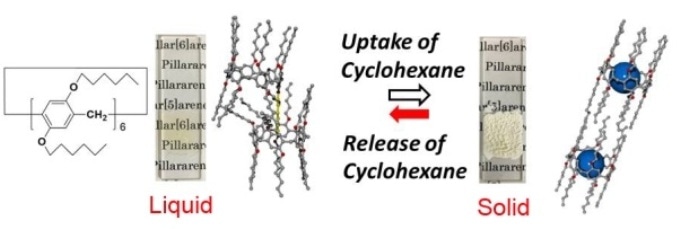 Figure 1. Schematic illustration of cyclohexane guest vapor-induced state change of pillar[6]arene with 12 n-hexyl groups.
Figure 1. Schematic illustration of cyclohexane guest vapor-induced state change of pillar[6]arene with 12 n-hexyl groups.
The transition between a solid and a liquid phase, a key process in daily life and materials science, is generally driven by a change in temperature or pressure. However, a reversible change of state caused by other stimuli is also possible: for example, light has been used to induce solid–liquid transitions.
Tomoki Ogoshi from Kanazawa University in Japan and colleagues studied pillar[n]arene molecules — pillar-shaped molecules that were first reported by their research group — to which functional groups can be added to modify their physical properties. Introducing 12 n-hexyl (C6H13) chains into the molecules transform the system into a room-temperature structural liquid, that is, a system with a certain degree of order at the nanoscale but without a periodic structure (Figure 1). The liquid solidifies when exposed to a guest vapor, whose molecules replace the n-hexyl substituents in the cavities of the pillar-shaped molecules. At the same time, the substituents located outside the cavities crystallize. The result is that, on a timescale of a few seconds, the system solidifies and the transparent liquid changes to a turbid solid.
As the competitive guest vapor the authors used cyclohexane, because it fits into the cavities of the pillar-shaped molecules and is easy to remove by heating the sample under reduced pressure, a process that results in the molecular system going back to the liquid state. The adsorption and desorption processes are characterized by nuclear magnetic resonance measurements, whereas the structure of the system is studied by X-ray diffraction.
The authors also investigated the uptake of other organic vapors by the structural liquid, observing that exposure to molecules that could be absorbed in the pores of the pillar-shaped molecules always resulted in a transition to a solid phase, whereas the phase transition was not observed for exposure to gases that had a low uptake by the structural liquid.
This system can be used as a detector of alkane vapors, an unusual device. “Because of the vapor selectivity, we postulate the vapor-induced state change can be applied for new vapor detection systems,” comment the authors. “Another application is adhesion materials using the guest vapor-induced state change.”
Background
Host-guest chemistry: supramolecular chemistry is a branch of chemistry that studies chemical systems composed by several molecules, describing their interaction, which are essential to many biological processes. Host-guest interactions are one example: this is the type of interaction at work when a host molecule forms a chemical compound with a guest molecule or ion without the involvement of covalent bonds. This type of interaction can be harnessed, for example, in drug delivery systems in which the presence of the host increases the solubility and availability of the drug (the guest).
Pillararenes: macrocycle molecules that take their name from their pillar shape. The cavities in these molecules can host electron-poor molecules. Some of these molecules have potential for biomedical applications, but also for gas absorption, ionic liquids and organic light-emitting materials.