Apr 2 2019
A research team at Sechenov First Moscow State Medical University employed 3D printing to develop biocompatible structures based on chitin derived from crab shells.
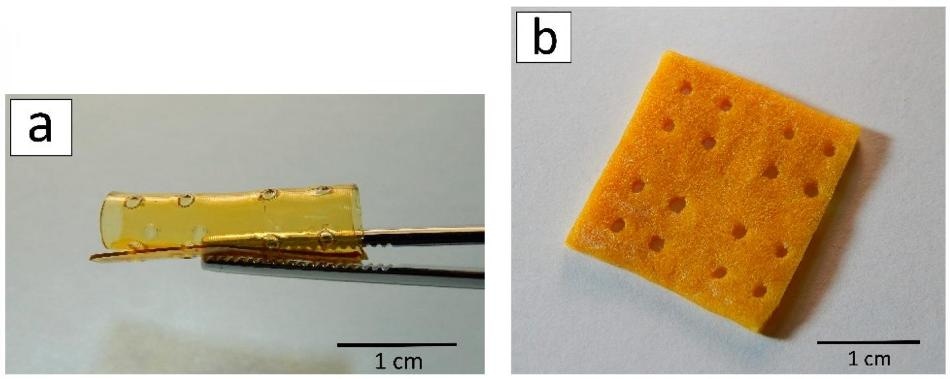 A 3D scaffold after laser stereolithography (a) and lyophilization (b). (Image credit: Ksenia Bardakova)
A 3D scaffold after laser stereolithography (a) and lyophilization (b). (Image credit: Ksenia Bardakova)
This technique will help create structures with specified shapes for a variety of biomedical applications, for example, the replacement of damaged soft tissues in the human body. The study was reported in Marine Drugs.
Shells and other by-products constitute 50% to 70% of the weight of all crabs entrapped across the globe. Usually, they are destroyed, which needs further investment. Only a negligible portion is processed. Nevertheless, the bodies of marine crustaceans consist of large amounts of chitin. This polysaccharide is prevalent in the wild, for instance, the exoskeletons of insects are composed of it. By eliminating the acetyl groups from chitin, one can get chitosan, which is a biopolymer with a distinctive set of physical, chemical, and biological properties.
It is biocompatible, which means no inflammation or immune response is caused by implanting it into the body. In addition, it has antimicrobial and antifungal properties and slowly decomposes without leaving any hazardous components in the body. Hence, chitosan and its derivatives hold potential for medicine. New kinds of biocompatible structures can be developed based on this to repair damaged tissues or as carriers for targeted drug delivery.
In the conventional method of extracting chitosan from chitin, the raw material is treated with aggressive chemical reagents like concentrated alkali solutions. These methods yield only a small amount of chitosan and the solutions used are toxic, hence they cannot be employed on the industrial scale. The authors of the article proposed a more eco-friendly approach of chitin modification known as mechanochemical synthesis. The technique involves three kinds of treatment of a solid mixture: with pressure, shear stress, and reagents. It uses less alkali when compared to conventional chemical synthesis and eliminates the need for using any catalysts, solvents, and process initiators. The chitosan produced in this manner may be used for medical purposes without purification and removal of left-over harmful components.
Using the same technique, the researchers produced many chitosan derivatives with different content of allylic groups (from 5% to 50%). During such modification, allylic groups (propylene derivatives, organic substituents with two bonds between carbon atoms) are incorporated into the structure of chitosan. This allows chitosan derivatives to create photo-bound films and 3D structures of any geometry in the presence of a photoinitiator and under the influence of laser and UV radiation.
The films composed of chitosan derivatives were produced via the photopolymerization technique: polymer solutions in acetic acid were kept on plastic and illuminated with UV light until they get hardened. To create 3D structures, the scientists employed a 3D printing technology known as laser stereolithography. It is a fast and straightforward 3D modeling technique without needing any costly equipment.
In accordance with a computer model, 3D scaffolds are created layer by layer. A photoinitiator was added to the solutions of chitosan derivatives, and subsequently, the photopolymerization reaction was started using a laser. The produced structures were first frozen and then dried in a vacuum chamber (this method is known as freeze-drying or lyophilization). Following this method, the material of the structures became porous.
On the last stage of the study, the group of researchers implanted the developed structures in rats (under the skin in the interscapular region). The experiment in vivo continued for 90 days, and any of the implants did not exhibit any signs of toxicity during this period. This proves that the scaffolds are biocompatible. The researchers discovered that the implanted structures began decomposing only after 60 days of the experiments. The group aims to learn how to control this process and to develop implants with necessary biodegradation speed.
This method of chitosan derivatives structuring provides the creation of 3D structures with physiologically relevant sizes. They can be used to heal large (more than 1 cm) tissue defects. Having studied the stability of the samples in vivo, we demonstrated for the first time that the areas of degradation are distributed periodically, not chaotically. It confirms the hypothesis regarding the mechanism of biodegradation of chitosan-based materials: the least ordered amorphous areas of the polymer degrade first. The understanding of this mechanism will help us form structures in which the rate of degradation would be comparable to the rate of restoration of the replaced tissue or organ. The scaffold would degrade in the precise amount of time that is required by the damaged tissue to restore its integrity and functions.
Ksenia Bardakova, Junior Research Associate, Department of Modern Biological Materials, Institute for Regenerative Medicine, Sechenov University
Ksenia Bardakova is also the co-author of the study.
The study is part of a research cycle on the creation of 3D structures from hydrogels (with water as the dispersion medium in which solid particles develop a 3D grid) on the basis of natural polysaccharides. The study is conducted by the researchers of Sechenov University along with their colleagues from the Institute of Photonic Technologies RAS, Institute of Synthetic Polymeric Materials of RAS, and the National University of Ireland Galway with the support of Russian Science Foundation. The partakers of the research also represented the Institute of Chemical Physics RAS and Baikal Institute of Environmental Management SB RAS.