Apr 22 2019
Traditional lithium-ion batteries are known to present certain risks of explosions and fire, unlike solid-state sodium-ion batteries that are relatively safer.
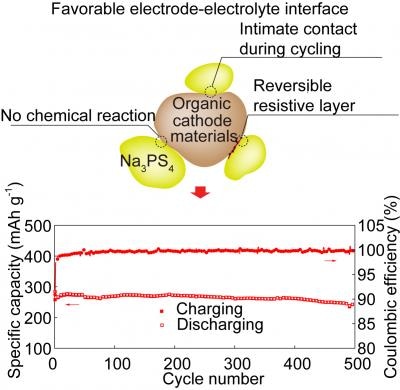 Forming compatible interfaces between cathode active materials and solid electrolytes is important for high-performance all-solid-state batteries. The organic cathode demonstrated here is (electro)chemically and mechanically compatible with a sulfide electrolyte. Its moderate redox potential enables the reversible formation of a resistive active material-electrolyte interface. (Image credit: University of Houston)
Forming compatible interfaces between cathode active materials and solid electrolytes is important for high-performance all-solid-state batteries. The organic cathode demonstrated here is (electro)chemically and mechanically compatible with a sulfide electrolyte. Its moderate redox potential enables the reversible formation of a resistive active material-electrolyte interface. (Image credit: University of Houston)
However, the performance of solid-state sodium-ion batteries has been extremely weak to compensate the safety benefits. Now, scientists have recently reported the development of a new organic cathode that considerably enhances energy density as well as stability.
The enhanced performance, featured in the journal Joule, is associated with two major findings:
- It is possible to reverse the resistive interface existing between the cathode and electrolyte that often forms at the time of cycling, thus prolonging the cycle life
- The organic cathode’s flexibility helped it to sustain a close contact with the solid electrolyte at the interface, even when the cathode contracted and expanded at the time of cycling.
According to Yan Yao, associate professor of electrical and computer engineering at the University of Houston and the paper’s corresponding author, the organic cathode—called PTO, short for pyrene-4,5,9,10-tetraone—provides exceptional benefits over earlier inorganic cathodes. However, the underlying principles are equally important, he stated.
We found for the first time that the resistive interface that forms between the cathode and the electrolyte can be reversed. That can contribute to stability and longer cycle life.
Yan Yao, Study Corresponding Author and Associate Professor, Department of Electrical and Computer Engineering, University of Houston
Yao is also a principal investigator at the Texas Center for Superconductivity at University of Houston. His research team concentrates on sustainable and green organic materials for the generation and storage of energy.
According to Yanliang “Leonard” Liang, a research assistant professor in the Department of Electrical and Computer Engineering at University of Houston, interface reversibility is the key, enabling the solid-state battery to achieve a greater energy density without affecting cycle life. Usually, the ability of a solid-state battery to store energy is stopped when there is a formation of the resistive cathode-electrolyte interface; when that resistance is reversed, energy density continues to be high at the time of cycling, he added.
Integrated with their liquid electrolytes, lithium-ion batteries are capable of storing comparatively large amounts of energy and are often used for powering the tools of contemporary life, ranging from hearing aids to cell phones. However, the risk of explosion and fire has attracted a great deal of interest in other kinds of batteries, and, in this regard, a solid-state sodium-ion battery provides the possibility of improved safety but at a relatively lower cost.
According to Xiaowei Chi, a post-doctoral researcher in Yao’s team, a major difficulty had been to identify a solid electrolyte that is just as conductive as the liquid electrolytes utilized in lithium-ion batteries. Now, with the availability of adequately conductive solid electrolytes, the solid interfaces were a remaining challenge.
A solid electrolyte raises one problem—the electrolyte finds it difficult to keep a close contact with a standard rigid cathode as the cathode contracts and expands at the time of battery cycling. PhD student Fang Hao, who is working in Yao’s team, informed that since the organic cathode is more pliable, it is able to stay in contact with the interface, thereby enhancing the cycling life. The contact continued to be stable through a minimum of 200 cycles, stated the researchers.
If you have reliable contact between the electrode and electrolyte, you will have a great chance of creating a high-performance solid-state battery.
Fang Hao, PhD Student, Department of Electrical and Computer Engineering, University of Houston
Apart from Yao, authors include co-first authors Hao and Chi, Liang, Ye Zhang and Hui Dong, all from the University of Houston UH; Kejie Zhao and Rong Xu of Purdue University; and Tanguy Terlier, Hua Guo, and Jun Lou of Rice University. Most of the study was funded by the U.S. Department of Energy’s Advanced Research Projects Agency-Energy (ARPA-E).