May 23 2019
Electricity has become a massive necessity in everyday life. However, the unrelenting need for electricity demands for progressively greener and "portable" energy sources. Although solar panels and windmills are encouraging alternatives, the variation in output levels based on external factors renders them undependable. Therefore, from the standpoint of resource allocation and economics, high-energy density secondary batteries are the answer.
 A unique method to use novel rock salt in rechargeable magnesium batteries. (Image credit: Tokyo University of Science)
A unique method to use novel rock salt in rechargeable magnesium batteries. (Image credit: Tokyo University of Science)
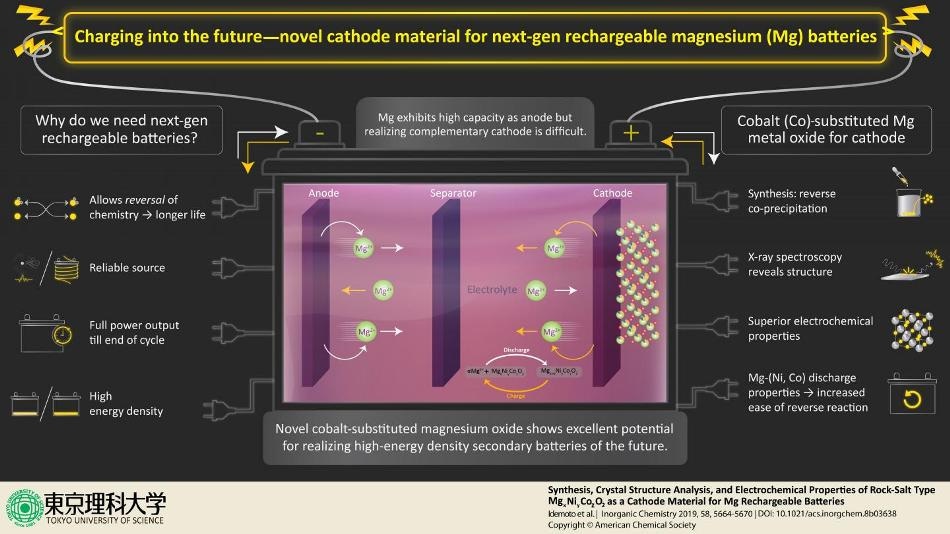
By synthesizing unique material (a metal compound) for electrode that allows reversing of the chemistry of ions, a team of scientists led by Prof. Idemoto from Tokyo University of Science fight the inefficient aspects of energy sources by laying a crucial foundation for the manufacture of next-generation rechargeable magnesium secondary batteries. The scientists are hopeful about the discovery and state, "We synthesized a rock salt type that has excellent potential for being used as the positive electrode material for next-generation secondary batteries."
The most widely used source of portable energy, a battery has three standard components—the cathode, the anode, and the electrolyte. These partake in an interplay of chemical reactions whereby the anode creates extra electrons (oxidation) that are absorbed by the cathode (reduction), leading to a process called redox reaction. Since the electrolyte hinders the flow of electrons between the cathode and anode, the electrons preferentially flow via an external circuit, thus introducing a flow of current or "electricity." When the material in the cathode/anode cannot absorb/shed electrons any more, the battery is considered useless.
However, some materials permit reversal of the chemistry, using external electricity that runs in the opposite direction, such that the materials may return to their primary state. Such rechargeable batteries are like the ones used in portable electronic devices such as tablets or mobile phones.
Prof. Idemoto and colleagues at Tokyo University of Science synthesized cobalt-substituted MgNiO2, which displays favorable results as an innovative cathode. "We focused on magnesium secondary batteries that use polyvalent magnesium ions as movable ions," states Prof. Idemoto while highlighting their study and its enticing potentials "which are expected to have high energy density in next-generation secondary batteries." Lately, the low toxicity of magnesium and the ease of performing reversed reactions have produced eagerness for utilizing it as anode material in high-energy density, rechargeable batteries. However, realization of this remains tough due to to the lack of an appropriate complementary electrolyte and cathode. This is precisely what these scientists are aiming to modify with their research published in the journal Inorganic Chemistry.
Grounded on standard laboratory methods, the scientists synthesized the novel salt using the "reverse co-precipitation" technique. From the aqueous solution, they could extract the novel rock-salt. To examine the structure as well as for lattice imaging of the extracted salt, they used neutron and synchrotron X-ray spectroscopy complementarily. In other words, they examined the diffraction patterns formed when the powder samples were irradiated with X-ray or neutrons, resulting in characteristic peaks in intensity at specific positions. At the same time, the scientists carried out theoretical calculations and simulations for the rock salt-types that revealed a probable "charge-discharge behavior" needed for ideal cathode materials. This allowed them to establish the arrangement of Mg, Ni, and Co cations in the rock-salt structure based on the most energetically steady structure among the 100 symmetrically produced distinct candidates.
Besides the structural analysis, the scientists also carried out charge-discharge tests with a tripolar cell and established reference electrodes, under several settings, to comprehend the electrochemical properties of the rock salt as a cathode material for the magnesium rechargeable batteries. They discovered that they could control the battery characteristics based on the Mg composition and the Ni/Co ratio. These electrochemical and structural analyses enabled them to reveal the best composition for the rock salt as a cathode material, together with its reliability under various ambient environments. Prof. Idemoto and the team are hopeful about the features of the synthesized rock salt, as they stress, "it has an excellent potential for use as the positive electrode material."
Right now, the secondary battery industry is controlled mainly by lithium ion batteries used for electricity storage, in portable devices and vehicles. There is, however, a cap on the storage and energy density of these batteries. But for Prof. Idemoto, limitations are only opportunities, as he maintains, "Novel magnesium secondary batteries have the potential to surpass and replace lithium ion batteries as high-energy density secondary batteries through future research and development."
With such positivity spewing from the study, one can confidently conclude that humans are charging into a tomorrow that is illuminated up by present-day science.