Aug 5 2019
A research work published in the August 2nd edition of Science reports that researchers have taken a great stride closer to achieving 3D bioprinting of functional organs, following the creation of a technique to rebuild components of the human heart.
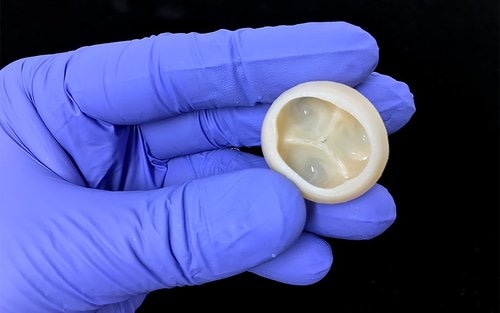 Trileaflet heart valve FRESH printed using collagen. (Image credit: Fluidform)
Trileaflet heart valve FRESH printed using collagen. (Image credit: Fluidform)
The group of scientists from Carnegie Mellon University created an advanced version of Freeform Reversible Embedding of Suspended Hydrogels (FRESH) technology to 3D print collagen with unparalleled complexity and build components of the human heart ranging from small blood vessels to valves to beating ventricles.
FRESH technology, which recently received the US patent 10,150,258, is now licensed to FluidForm, a startup dedicated to the radical extension of the potential of 3D printing.
We now have the ability to build constructs that recapitulate key structural, mechanical, and biological properties of native tissues. There are still many challenges to overcome to get us to bioengineered 3D organs, but this research represents a major step forward.
Prof. Adam Feinberg, CTO and Co-Founder, FluidForm
Feinberg is also the Principal Investigator of the Regenerative Biomaterials and Therapeutics Group at Carnegie Mellon, where the study was carried out.
While 3D bioprinting has reached significant milestones, direct printing of soft biomaterials and living cells has been complex to achieve. One of the main challenges is supporting soft and dynamic biological materials during the printing process to attain the fidelity and resolution needed to reconstruct complex 3D structure and function.
FRESH employs an embedded printing method that overcomes this obstacle by using a temporary support gel, thus facilitating 3D printing of complex scaffolds using collagen in its native unmodified form. Earlier, scientists were restricted due to difficulty in printing soft materials with high fidelity beyond a few layers in height because of sag.
The nine members of the Carnegie Mellon group headed by Andrew Lee and Andrew Hudson, co-first authors and FluidForm co-founders, solved these issues by devising a method that taps rapid pH change to induce collagen self-assembly.
The FRESH 3D bioprinted hearts were developed on the basis of human MRI and precisely simulated patient-specific anatomical structure. Smaller cardiac ventricles printed with human cardiomyocytes revealed directional action potential propagation, synchronized contractions, as well as wall-thickening up to 14% during peak systole.
However, challenges continue to persist, for example, producing the billions of cells needed to 3D print larger tissues, realizing manufacturing scale, and still undefined regulatory process for clinical translation.
Although the human heart was employed for proof-of-concept, FRESH printing of collagen and other soft biomaterials is a platform that has the ability to construct advanced scaffolds for a broad variety of tissues and organ systems.
FluidForm is extraordinarily proud of the research done in the Feinberg lab. The FRESH technique developed at Carnegie Mellon University enables bioprinting researchers to achieve unprecedented structure, resolution, and fidelity, which will enable a quantum leap forward in the field. We are very excited to be making this technology available to researchers everywhere.
Mike Graffeo, CEO, FluidForm
FluidForm is commercializing FRESH technology through its first product, LifeSupport™ bioprinting support gel, allowing scientists worldwide access to high-performance 3D bioprinting of cells, collagen, and a broad variety of biomaterials.