Nov 11 2019
According to researchers at King Abdullah University of Science and Technology (KAUST), expanding the list of organic ingredients comprising perovskite solar materials can possibly improve their performance and long-term stability.
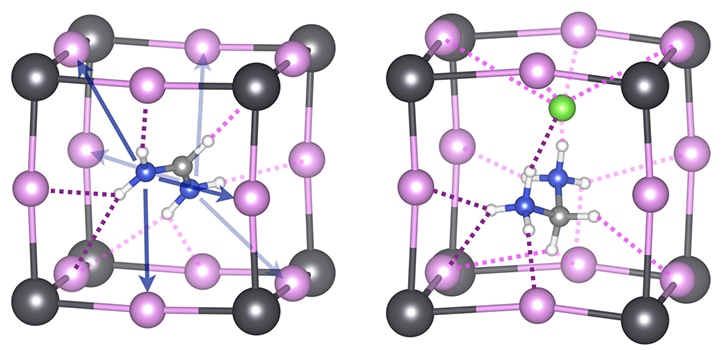 Hybrid perovskites are an effective and relatively inexpensive solar cell material but lag behind silicon in terms of stability. The pink and grey components represent the inorganic perovskite skeleton with incorporated organic cations. Image credit: © 2019 Aleksandra Oranskaia.
Hybrid perovskites are an effective and relatively inexpensive solar cell material but lag behind silicon in terms of stability. The pink and grey components represent the inorganic perovskite skeleton with incorporated organic cations. Image credit: © 2019 Aleksandra Oranskaia.
Recently, perovskite materials have gained much attention in solar materials studies because of their ability to harvest solar energy virtually as efficiently as traditional silicon solar cells. These materials can also be produced more easily and cost-effectively.
However, perovskite materials still lag behind silicon because of their poor long-term stability. Now, a potential solution has been identified by a research team that included Udo Schwingenschlögl from the KAUST Solar Center and Aleksandra Oranskaia, his PhD student.
The extensively researched perovskite materials for solar applications include a negatively charged lead-halide inorganic skeleton, together with positively charged organic cations, like formamidinum (FA) or methylammonium (MA). These collectively combine into a highly regular arrangement of atoms.
The organic component provides structural stability and thus plays a more supporting role, while the lead halide component is largely responsible for interacting with light. But the comparatively poor stability of these materials continues to limit their development at the commercial level.
With the help of computational modeling, both Oranskaia and Schwingenschlögl investigated the organic component of solar perovskite materials, searching for ways to enhance the stability of FA-lead halide perovskites.
Our motivation was to apply new computational methods to one of the hottest problems in the field of perovskite solar cells.
Aleksandra Oranskaia, PhD Student, KAUST Solar Center, King Abdullah University of Science and Technology
Experimental research has demonstrated that FA-based perovskites are more stable when compared to MA. Hence, the researchers initially compared the bonding strengths of FA and MA, paying attention to noncovalent forms of bonding like hydrogen bonding. Next, they investigated whether the stability of the FA-lead halide perovskite structure can be further improved by adding other organic “dopants” into it.
We showed for the first time that the noncovalent bonding strength of organic cations can be used to improve hybrid perovskite materials.
Udo Schwingenschlögl, Professor, KAUST Solar Center, King Abdullah University of Science and Technology
Even though covalent bonds are known to be the strongest, other types of covalent bonds, such as halogen bonding and hydrogen bonding between the lead halide component and the organic cation dopants, help in stabilizing the structure of the perovskites.
“We show that doping with organic cations of the right volume and shape—those that bond more strongly than FA to the inorganic skeleton via hydrogen and halogen bonding—can stabilize the material,” added Oranskaia.
Organic cations—with noncovalently and covalently bound chlorine ions or atoms—have been demonstrated to be specifically effective. These cations help to suppress the destructive movements of halides called X-migrations.
This offers a strategy to boost the performance of lead halide solar cells.
Udo Schwingenschlögl, Professor, KAUST Solar Center, King Abdullah University of Science and Technology
Schwingenschlögl added that the team is now planning to investigate how the phase stability of other solar-associated materials is affected by noncovalent interactions.