Mar 13 2020
At Duke University, biomedical engineers have developed a new technique for making microparticles that are harmless to living tissues. This method will enable the researchers to create novel shapes that could be used for tissue engineering, diagnostics, and drug delivery.
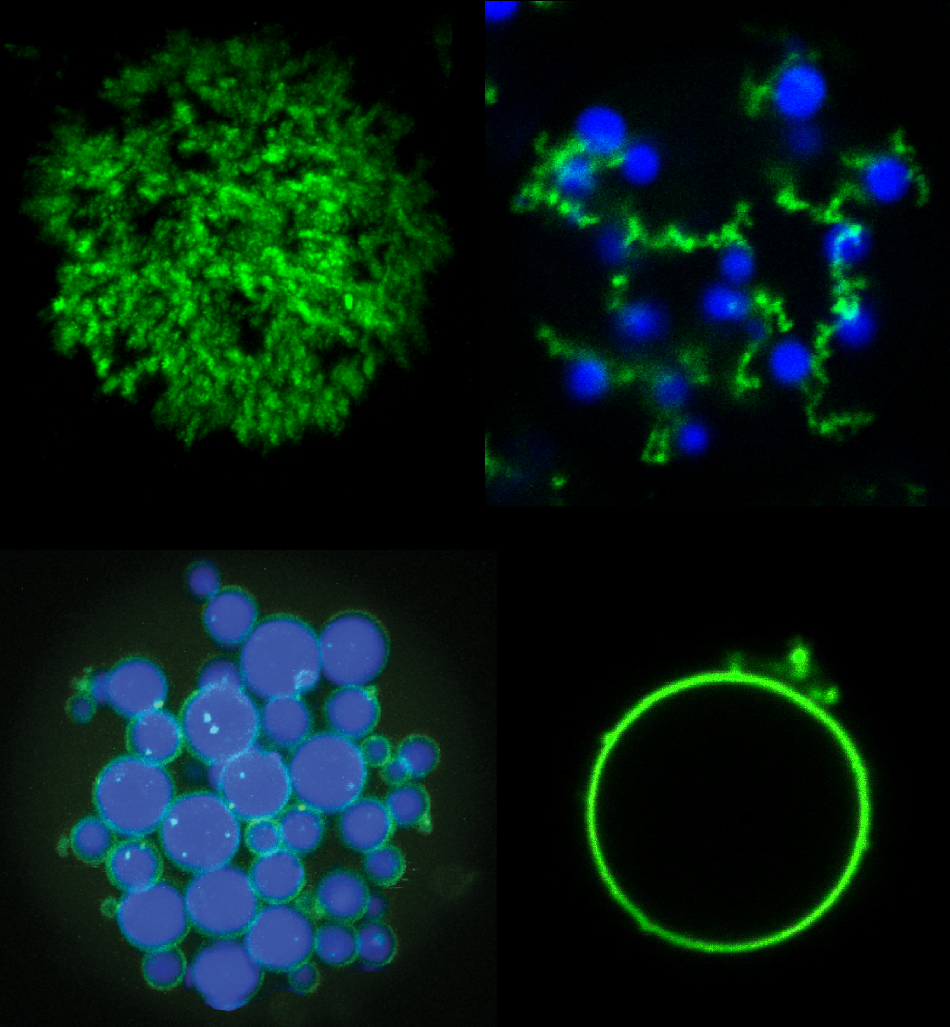 Mixtures of POPs (green) and ELPs (blue) can be used to create a variety of new microparticle architectures including (clockwise from upper left) porous particles, “fruits-on-a-vine” networks, single hollow “vesicle-like” particles, and core-shell networks. Image Credit: Stefan Roberts, Duke University.
Mixtures of POPs (green) and ELPs (blue) can be used to create a variety of new microparticle architectures including (clockwise from upper left) porous particles, “fruits-on-a-vine” networks, single hollow “vesicle-like” particles, and core-shell networks. Image Credit: Stefan Roberts, Duke University.
The study results were recently published online in the Nature Communications journal on March 12th, 2020.
With nothing more than some heat and light, we can make some pretty bizarre microparticles. The technique is simple enough that it could be scaled up to make billions of microparticles in a matter of minutes.
Stefan Roberts, Research Scientist, Biomedical Engineering, Duke University
In the realm of biocompatible microparticles, factors like internal microstructure, size, shape, and type of material govern their inherent properties.
While many complex microparticles can already be produced by research laboratories and companies, the process often involves advanced manufacturing methods like flow lithography or multiple-emulsion microfluidics. But both these methods come with certain disadvantages.
Although multiple-emulsion microfluidics intensively regulates a set of individual oil droplets, it does not effectively separate the materials from each other. Moreover, it cannot be utilized for commercial-scale production.
By irradiating light via a patterned mask, the flow lithography technique etches shapes in soft materials and can produce several particles quickly. However, the procedure is too complicated to adapt to internal architectures and intricate shapes.
In association with Ashutosh Chilkoti, the Alan L. Kaganov Distinguished Professor of Biomedical Engineering at Duke University, Roberts embarked to experiment with an entirely new approach, that is, using biological materials.
Both researchers have been working with elastin-like polypeptides (ELPs) for a long time. ELPs can be defined as disordered proteins that, just like a spaghetti ball, obtain their stability from disorder and do not have any real shape.
Of late, the researchers started to work with partially ordered proteins (POPs), which not only maintain most of the biologically useful characteristics of ELPs but also have sufficient ordered segments to offer more stability than wet noodles.
These two protein types can be designed to shift back and forth between phase states at specific temperatures. While this is a handy feature for applications, like supporting the growth of tissues in wounds or slowly discharging drugs into the body, the team soon found out that different shapes of particles could also be produced by putting POPs and ELPs together.
Disordered proteins are a hot topic in biology, with many researchers trying to discover how proteins without shape can still have a biological purpose. An undercurrent of our work is to instead think of these proteins as a materials scientist would and see if we can engineer them for our own biological functions in ways that can’t be achieved with current materials.
Stefan Roberts, Research Scientist, Biomedical Engineering, Duke University
Both Roberts and Chilkoti demonstrated in the study how certain new microparticles were developed using these two types of proteins. By adjusting the temperatures at which the proteins disassemble and assemble, and sweeping back and forth through different temperatures at different rates, the scientists demonstrated that they have the potential to produce a series of shapes like a shell without core, a shell with a solid core, and even a tangle of cords sprinkled with shells that they called “fruits on a vine.”
Then, by integrating photosensitive amino acids, the researchers also demonstrated that these shapes can be frozen into solid microparticles using a flash of light. According to them, the potential to produce tiny particles with accurately isolated regions is applicable for applications like tissue engineering and drug delivery.
Every set of parameters concurrently produces an unlimited number of biocompatible, solid microparticles that are somewhat larger than a normal cell. It just takes a few minutes, and all this occurs in a quantity of liquid that is roughly the size of a water droplet.
This is a test case for a type of material that is flexible and simple enough to create both commonly used shapes and architectures that aren’t seen using current techniques. We’re using new biocompatible materials to create never-before-seen shapes simply by heating, cooling and shining a light on them.
Stefan Roberts, Research Scientist, Biomedical Engineering, Duke University
The study was supported by the National Science Foundation’s Research Triangle MRSEC (DMR-11-21107), the National Institutes of Health (R35GM127042), Graduate Research Fellowship Program (1106401), and Analytical Instrumentation Facility at NC State University (ECCS-1542015).