Aug 26 2020
A research team from Penn State reports that a self-assembling, thin layer of electrochemically active molecules could be the solution in the hunt for a fast-charging, reliable, cold-weather battery for automobiles.
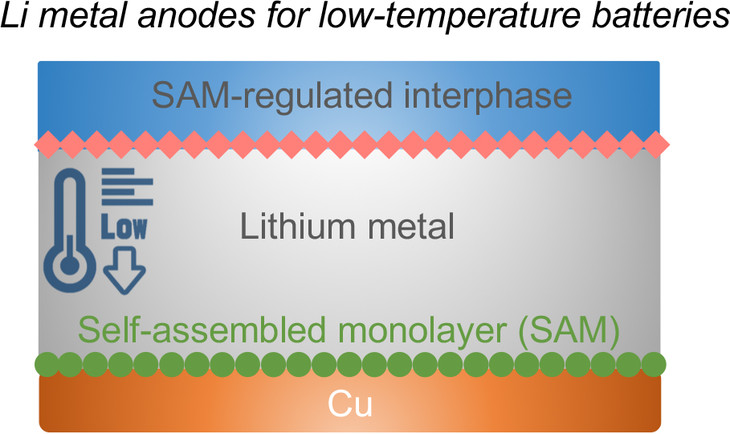 Image of the layers in a lithium metal anode for low-temperature batteries. Image Credit: Donghai Wang, Penn State.
Image of the layers in a lithium metal anode for low-temperature batteries. Image Credit: Donghai Wang, Penn State.
The lithium metal battery is the next generation of battery after the lithium ion battery. It uses a lithium anode and has higher energy density, but has problems with dendritic growth, low efficiency and low cycle life.
Donghai Wang, Professor of Mechanical Engineering, Battery and Energy Storage Technology Center, Penn State
Wang a key researcher in the Battery and Energy Storage Technology Center.
According to the researchers, the solution to these challenges is a self-assembling monolayer that is electrochemically active and can disintegrate into its proper components and safeguard the surface of the lithium anode.
The battery is made of a lithium metal oxide cathode, the lithium anode, and an electrolyte that also includes lithium-ion conducting materials and the protective, thin-film layer.
In the absence of the protective layer, lithium crystal spikes would grow in the battery upon rapid charging or under cold conditions. Ultimately, these lithium spikes tend to short out the battery, considerably affecting the cycle life and usefulness of the battery.
The key is to tune the molecular chemistry to self-assemble on the surface. The monolayer will provide a good solid electrolyte interface when charging, and protect the lithium anode.
Donghai Wang, Professor of Mechanical Engineering, Battery and Energy Storage Technology Center, Penn State
The monolayer was deposited on a thin copper layer by the researchers. Upon charging the battery, lithium strikes the monolayer and disintegrates to develop a stable interfacial layer. Some part of the lithium gets deposited on the copper together with the remaining layer, and the disintegrated part of the original layer reforms over the lithium, safeguarding the lithium and inhibiting the formation of lithium dendrites.
The researchers of the study note that this technology can improve the storage capacity of the battery and can increase the number of times the battery can be charged. But at this stage, the battery can be charged only a few hundred times. The study was published recently in the latest issue of Nature Energy.
The key is that this technology shows an ability to form a layer when needed on time and decompose and spontaneously reform so it will stay on the copper and also cover the surface of the lithium. Eventually it could be used for drones, cars, or some very small batteries used for underwater applications at low temperatures.
Donghai Wang, Professor of Mechanical Engineering, Battery and Energy Storage Technology Center, Penn State
Other researchers who were part of this researchers were postdoctoral scholars Yue Gao and Shua Liu, and graduate students Daiwei Wang and Tianhang Chen from Penn State, all belonging to Donghai Wang laboratory; and Ke Wang and Haiying Wang, technical staff members at the Materials Research Institute.
Tomas Rojas, graduate student, Ohio University; and Anh T. Ngo, assistant physicist, Argonne National Laboratory and associate professor, chemical engineering, the University of Illinois at Chicago also contributed to the study.
This study was supported by the U.S. Department of Energy.
Journal Reference:
Gao, Y., et al. (2020) Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nature Energy. doi.org/10.1038/s41560-020-0640-7.