Oct 19 2020
At the Massachusetts Institute of Technology, chemical engineers have developed a new system that could offer a means to constantly eliminate carbon dioxide from a flow of waste gases, or even from the atmosphere.
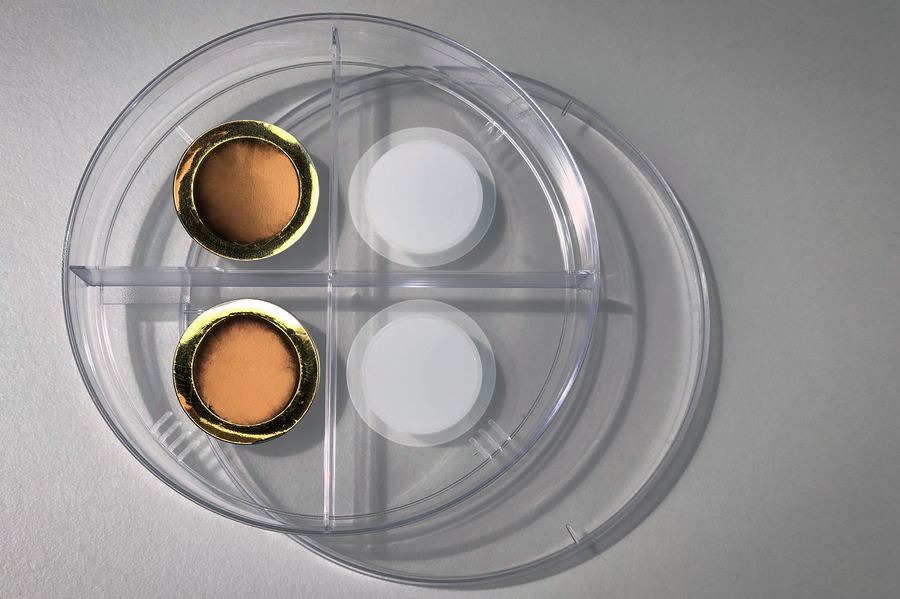 On the right is a porous anodized aluminum oxide membrane. The left side shows the same membrane after coating it with a thin layer of gold, making the membrane conductive for electrochemical gas gating. Image Credit: Felice Frankel.
On the right is a porous anodized aluminum oxide membrane. The left side shows the same membrane after coating it with a thin layer of gold, making the membrane conductive for electrochemical gas gating. Image Credit: Felice Frankel.
The main component of the system is an electrochemically supported membrane, the permeability of which to gas can be altered at will, with no moving parts and comparatively little energy usage.
The membranes have been developed using anodized aluminum oxide, feature a honeycomb-like structure with hexagonal openings that enable gas molecules to stream in and out when it is in the open state.
But the passage of gas can be stopped by electrically depositing a thin metal layer to cover the pores of the membrane. The study was recently published in the Science Advances journal, in a paper authored by Professor T. Alan Hatton, postdoc Yayuan Liu, and four others.
According to the research team, the new mechanism called “gas gating” could be used for the constant elimination of carbon dioxide from a series of industrial exhaust streams and ambient air. They have constructed a proof-of-concept device to demonstrate this process in action.
The device makes use of a redox-active carbon-absorbing material, interspersed between two switchable gas gating membranes. The gating membranes and the sorbent are in close contact with each other and are submerged in an organic electrolyte to offer a medium for zinc ions to shuttle back and forth.
It is possible to open or close both the gating membranes electrically by interchanging the polarity of a voltage between them, which results in the shuttling of zinc ions from one side to another. At the same time, the ions block one side by developing a metallic film over it and opening the other by dissolving its film away.
When the sorbent layer is open to the side through which the waste gases flow, the material immediately soaks up carbon dioxide until reaching its capacity. Then, the voltage can be interchanged to block off the feed side and open the other side through which a concentrated stream of almost pure carbon dioxide is discharged.
The development of a system with alternating membrane sections working in opposite phases would enable continuous operation in a setting like an industrial scrubber. At any given time, half of the sections would be absorbing the gas and the other half would be discharging it.
That means that you have a feed stream coming into the system at one end and the product stream leaving from the other in an ostensibly continuous operation.
T. Alan Hatton, Professor, MIT
“This approach avoids many process issues” that would be involved in a conventional multicolumn system, where adsorption beds in turn need to be shut down, purged, and regenerated, prior to being exposed again to the feed gas to start the next adsorption cycle.
The purging steps are not needed in the newly developed system, and the steps all take place purely inside the unit itself. The main innovation of the researchers was to use electroplating as a method to open and close the pores present in a material.
During the study, the researchers had attempted a range of other methods to reversibly close pores in a membrane material, like the use of small magnetic spheres that could be placed to block funnel-shaped openings, but these other techniques did not prove to be highly efficient.
Specifically, metal thin films could be effective as gas barriers, and the ultrathin layer utilized in the new system needs the least amount of the zinc material, which is copious and economical.
It makes a very uniform coating layer with a minimum amount of materials.
Yayuan Liu, Postdoc, MIT
One major benefit of the electroplating technique is that as soon as the condition changes, whether in the open or closed position, it does not need any energy input to preserve that state. Energy is needed just to switch back again.
Such a system could prospectively make an essential contribution toward restricting greenhouse gas emissions into the air, as well as direct-air capture of carbon dioxide emitted earlier.
Hatton thinks that while the main focus of the researchers was on the challenge of splitting up carbon dioxide from a stream of gases, the system could be adapted to an extensive range of chemical separation and purification processes.
We’re pretty excited about the gating mechanism. I think we can use it in a variety of applications, in different configurations. Maybe in microfluidic devices, or maybe we could use it to control the gas composition for a chemical reaction. There are many different possibilities.
T. Alan Hatton, Professor, MIT
The researchers involved in the study were graduate student Chun-Man Chow, postdoc Katherine Phillips, and recent graduate’s Miao Wang Ph.D. ’20 and Sahag Voskian Ph.D. ’19. This study was supported by ExxonMobil through the MIT Energy Initiative.
Journal Reference:
Liu, Y., et al. (2020) Electrochemically mediated gating membrane with dynamically controllable gas transport. Science Advances. doi.org/10.1126/sciadv.abc1741.