Nov 25 2020
The development of safe and sustainable nuclear energy fuels forms an essential part of Los Alamos National Laboratory’s energy security mission.
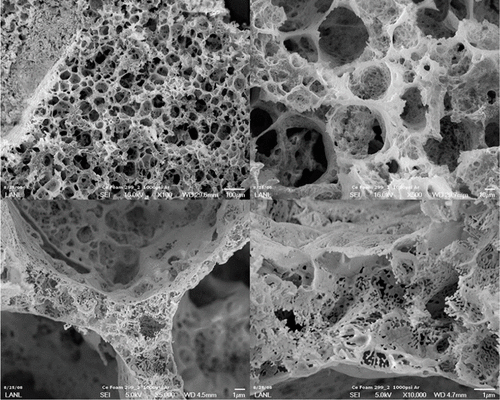 Scanning electron microscope images of cerium nitride foam. Image Credit: Los Alamos National Laboratory.
Scanning electron microscope images of cerium nitride foam. Image Credit: Los Alamos National Laboratory.
In existing nuclear power plants, a radioactive actinide oxide called uranium dioxide is most extensively used as nuclear fuel. A novel “combustion synthesis” process developed recently for lanthanide metals—non-radioactive and positioned one row above actinides on the periodic table—could help produce sustainable and safe nuclear fuels.
Actinide nitride fuels are potentially a safer and more economical option in current power-generating systems. Nitride fuels are also well suited to future Generation IV nuclear power systems, which focus on safety, and feature a sustainable closed reactor fuel cycle. Actinide nitrides have superior thermal conductivity compared to the oxides and are significantly more energy dense.”
Bi Nguyen, Study Lead Author and Agnew Postdoc, Los Alamos National Laboratory
The study was published recently in the Inorganic Chemistry journal and chosen as an American Chemical Society Editors’ Choice Featured Article.
Nitrides are a group of chemical compounds made of nitrogen, in contrast to oxides, which are made of oxygen.
Thanks to their energy density, actinide nitride fuels offer more sustainability and safety, providing more energy from less material and improved thermal conductivity. All these features enable lower temperature operations, providing them a huge margin to meltdown under abnormal conditions.
However, synthesizing actinide nitrides is very difficult, and the production of huge amounts of high-purity actinide nitrides remains a significant obstacle to their use.
Lanthanides and actinides are added at the bottom of the periodic table, and possible techniques to produce actinide materials are normally first tested with the lanthanides since they behave in a similar way but are not radioactive.
Researchers from Los Alamos National Laboratory and Naval Research Laboratory have identified that LnBTA [lanthanide bis(tetrazolato)amine] compounds could be burned to generate high-purity lanthanide nitride foams through a special method known as combustion synthesis.
This technique involves using a laser pulse to activate dehydrated LnBTA complexes, which are further exposed to a self-sustained combustion reaction in an inert atmosphere to synthesize nanostructured lanthanide nitride foams. This study was financially supported by the Laboratory Directed Research and Development (LDRD) program.

Combustion synthesis of LnBTA compound. Video Credit: Los Alamos National Laboratory.
LnBTA compounds are very simple to prepare in bulk, and their combustion is instantly scalable. The Laboratory’s Weapons Modernization and Chemistry divisions have been in collaboration to analyze actinide analogs for combustion production of actinide nitride fuels.
The research group members are Nguyen, David Chavez, Bryce Tappan, and Alexander Mueller of the Explosives Science and Shock Physics group; Jacqueline Veauthier and Jaqueline Kiplinger of the Inorganic Isotope and Actinide group; Brian Scott of Materials Synthesis and Integrated Devices group; and Damon Parrish of the Naval Research Laboratory.
Journal Reference:
Nguyen, T.-A. D., et al. (2020) Lanthanide Complexes of Bis(tetrazolato)amine: A Route to Lanthanide Nitride Foams. Inorganic Chemistry. doi.org/10.1021/acs.inorgchem.0c02480.